
Enhanced recovery after surgery (ERAS) in patients treated with radical cystectomy
Introduction
Bladder cancer is one of the most common malignancies worldwide (1). According to European Association of Urology (EAU) guidelines, the standard of care in muscle invasive bladder cancer (MIBC) is radical cystectomy (RC) supplemented with systemic chemotherapy. Moreover, RC is also recommended in some high and very high-risk cases of non-muscle invasive bladder cancer (NMIBC), as well as in bacillus Calmette-Guérin (BCG) failure (2). This makes the RC to be firmly constituted in the treatment of bladder cancer. Yet, there is another side of the coin. RC is a very mutilating operation during which a large part of the pelvic tissues and lymph nodes are removed. Additionally, in most cases, some parts of bowel are needed for urinary diversion. Because of that the RC is a morbid procedure with very high risk of complications, including death.
Lowering complications risk associated with RC is subject of considerable interest. Many evidence-based data about improvements in operative technique, anaesthetic management and patient care have been published and fast-track and enhanced recovery programs were created. In 2013, Enhanced Recovery After Surgery (ERAS) society published coordinated perioperative RC guidelines with aim to fight surgical dogma and tradition, reduce surgical stress, and finally, facilitate postoperative recovery, hasten return to normal activities and improve quality of life (QoL) (3). Nevertheless, a significant portion of original recommendations are based on low quality data or are extrapolated from general surgery.
The aim of this study was to present a review of up-to-date literature in patients treated with RC analysing ERAS influence on peri- and post-operative complications, costs and patient related outcomes.
Evidence acquisition
A systematic literature search according to PRISMA guidelines within the Medline, Embase, and Web of Science databases was conducted in September 2019 for papers presenting the ERAS protocol in RC setting year 2013 (after publication of ERAS guidelines) as the beginning of the search, using the terms “cystectomy” in conjunction with enhanced recovery after surgery, ERAS, complication, management. Boolean operators (NOT, AND, OR) were employed to extend the search (Figure 1). AutoAlerts in Medline were also searched, as well as reference lists of used articles. The search was limited to English literature. Articles that did not address the topics were excluded.
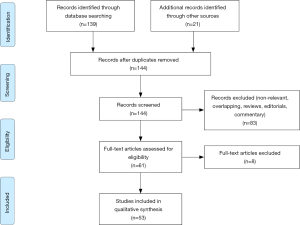
Evidence synthesis
RC morbidity—ERAS rationale
RC, especially when performed in palliative setting, is associated with high complication and readmission rates, as well as perioperative mortality. This is caused by complex nature of the surgery itself (urinary diversion included) and is also related to patient’s comorbidities status. Current reports state that severe RC complications applies to about one-third of patients during hospitalization and more than two-thirds of cases within 3 months postoperatively (4-14). Additionally, 2–4% of patients die due to surgery (4,15).
Complications after RC include gastro-intestinal problems (30%), infections (25%), complications related to the wound and abdominal stoma (15%), upper urinary tract issues (11%), cardiovascular disorders (11%) and venous thromboembolism (8%) (16). What is more, various metabolic changes such as bowel function abnormalities, malabsorption of various vitamins, acid-base and electrolyte imbalance, abnormalities bone metabolism dysfunctions, formation of urinary stones, and disturbances in kidneys or liver function are observed (17,18).
Main pillars of ERAS for RC (3)
Preoperative items
Preoperative counselling and education
Majority of patients undergo RC with urinary diversion related to abdominal stoma. Therefore, preoperative and postoperative “stoma education” should be emphasized to decreases anxiety and shorten the hospital stay. It has been shown in surgical population that preoperative psychological intervention and meticulous counselling with detailed information may further diminish fear and anxiety, enhance postoperative recovery and quicken hospital discharge.
Evidence for RC/grade of recommendation: not available/strong.
Preoperative medical optimization
As RC is associated with severe physiological stress, equalization and optimization of metabolic comorbidities and malnutrition, cessation of smoking/alcohol intake/drug use and implementation of physical exercise (prehabilitation) are strongly recommended (19,20). It is especially evident in malnourished patients and patient with preoperative anaemia (21).
Evidence for RC/grade of recommendation: not available/strong.
International Consensus Statement on the Perioperative Management of Anaemia and Iron Deficiency guidelines state that patients undergoing major surgery, where estimated blood loss is more than 500 mL, require treatment of iron deficiency (regardless of anaemia presence) (22). Recent studies also present improved postoperative outcomes after planed preoperative oral nutrition supplementation or immunonutrition (21,23,24).
Preoperatively, identification of patients with high complication risk may be performed used validated scoring systems [e.g., the NCEPOD Surgical Outcome Risk Tool (SORT), The American College of Surgeons Mortality and Morbidity Risk Calculator, the P-POSSUM, the Lee’s Cardiac Risk Index] (25). Also, preoperative cardiopulmonary exercise testing and frailty assessment are recommended before extensive pelvic surgery (26,27).
Oral mechanical bowel preparation
Bowel preparation was performed before intestinal surgery to reduce the theoretical risk of postoperative infectious complications by decreasing the bacterial load. However, there are no data proving that phenomenon. Nowadays, piling evidence show that bowel preparation may be safely omitted without increasing the risk of complications. Also, it has to be highlighted, that bowel preparation is associated with intestinal mucosal architectural change and electrolyte disturbance—both significantly influencing postoperative recovery (28). Therefore, none bowel preparation is recommended before RC.
Evidence for RC/grade of recommendation: moderate/strong.
Preoperative carbohydrate loading and preoperative fasting
Preoperative carbohydrate loading was shown to reduce thirst, insulin resistance and to help preserving lean body mass and muscle strength after intestinal surgery. Also, there is no solid data showing any benefit in abstaining from solid food for more than 6 hours before anaesthesia (theoretical risk of pulmonary aspiration). Consequently, carbohydrate-rich fluids are recommended up to 2 hours before anaesthesia.
Evidence for RC/grade of recommendation: not available/strong.
Pre-anaesthesia medications
It is recommended to use short-acting (instead of long-acting ones) sedation to improve postoperative recovery and mobilization, and to avoid cognitive impairment especially in the elderly population.
Evidence for RC/grade of recommendation: not available/strong.
Thrombo-embolic prophylaxis
Thromboprophylaxis ought to be used up 1 month after the operation to decreases the incidence of symptomatic deep vein thrombosis. There is no evidence to support the hypothesis of increased risk of clinically important bleeding with prolonged thromboprophylaxis. What is more, compressive stockings and intermittent pneumatic compression devices should be employed.
Evidence for RC/grade of recommendation: not available/strong.
Intraoperative items
Epidural analgesia
The use of the epidural analgesia for 48–72 hours allows for provision of superior pain relief (with subsequent opioid thrift), faster functional recovery and therefore reduction in cardiopulmonary complications.
Evidence for RC/grade of recommendation: not available/strong.
Minimally invasive approach and minimal resection site drainage
Laparoscopic or robotic approach in pelvic surgery were proved to be associated with various advantages including lower inflammatory response and lower complication rate when compared with open approach. Available data for robotic RC (low and moderate quality) show reduced overall perioperative complications risk, longer operative time and shorter length of stay (LoS), with comparable short-term oncological results. Yet, without good quality data, authoritative recommendations cannot be given (29,30).
Evidence for RC/grade of recommendation: low/strong.
It was proven in gastrointestinal surgery that avoidance of suction-drainage of the peritoneal cavity is not related in higher complication risk. Yet, because of lack of papers in RC setting (in the presence of potential urinary leak) those recommendations might not apply to bladder cancer patients.
Evidence for RC/grade of recommendation: not available/weak.
Antimicrobial prophylaxis
Perioperative antibiotics should be administered before skin incision and less than 1 h before surgery and should be effective against both aerobes and anaerobes. If there are no specific guidelines based on local epidemiological data, 2nd/3rd generation cephalosporin/aminopenicillins + metronidazole is recommended.
Evidence for RC/grade of recommendation: not available/strong.
Anaesthetic protocol
According to ERAS guidelines thoracic epidural analgesia, minimizing opioids use, short-acting anaesthetic agents and prevention of hypoxia and hypothermia are strongly recommended. Additional attention should be paid to maintain normoglycemia and normovolemia—blood loss is limited by controlled hypotension, antifibrinolytics, and timely substitution of blood loss. What is more, lung ventilation with low tidal volumes to limit peak airway pressures should be employed.
Evidence for RC/grade of recommendation: not available/strong.
Perioperative fluid management
Proper fluid management is essential to provide suitable visceral blood flow and to minimize the risk of complications, especially in high risk population. In the available literature, goal directed fluid therapy using oesophageal Doppler to achieve “near maximal stroke volume” has been recommended in pelvic surgery. Yet, those reports are based mainly on low risk rectal surgery patients.
Evidence for RC/grade of recommendation: low/strong.
Studer et al. propose low-dose perioperative norepinephrine administration together with intraoperative restrictive hydration. Additionally, less fluid may be given during lymphadenectomy and cystectomy, and more fluid can be administered during reconstructive part of the operation. These manoeuvres significantly lowered intraoperative blood loss, blood transfusions rate, the risk of gastrointestinal disturbances and LoS. Concurrently, authors have not observed increased risk of infectious and cardiovascular complications, or tissue hypoperfusion (17,31-33).
Recent reports also proved safety and efficiency of intraoperative cell salvage transfusion (34).
Moreover, in the international trial on 3,000 randomly assigned patients undergoing major abdominal surgery receiving a restrictive or liberal intravenous-fluid regimen during and up to 24 hours after surgery, it was shown that the rate of acute kidney injury was significantly higher in the restrictive fluid group. The rate of septic complications or death, rates of surgical-site infection and renal-replacement therapy were higher in the restrictive fluid group, but not statistically significant (35). Similar results were achieved in recent study on RC (36).
Postoperative items
Nasogastric intubation
According to available literature, early removal of nasogastric intubation after RC is not associated with increased risk of complications, prolonged LoS and recovery of bowel transit. Therefore, prolongation of nasogastric intubation is not recommended.
Evidence for RC/grade of recommendation: low/strong.
Urinary drainage
Low quality data suggest that ureteral stenting results in improved drainage of the upper urinary tract, improved bowel recovery and reduced occurrence of metabolic acidosis.
Evidence for RC/grade of recommendation: vert low/weak.
Prevention of postoperative ileus, nausea and vomiting
Various factors including fluid monitoring, minimalized approach, ureteral stenting and opioid-sparing analgesia were shown to have influence on reducing postoperative ileus, nausea and vomiting. Trials assessing influence of chewing gum proved shorter time to flatus and first bowel movement, however, failed to show statistical difference in postoperative morbidity and LoS (37). Also, some reports show that the use of metoclopramide reduced rates of nausea and vomiting and possibly gastrointestinal complications (38).
In cases with high risk of nausea occurrence (non-smokers, female patients, patients with a history of motion sickness and opioids users) a multimodal anti-emetic prophylaxis should be adopted.
Evidence for RC/grade of recommendation: very low to moderate/strong.
Postoperative analgesia
Multimodal opioid-sparing together with epidural analgesia is recommended to provide superior pain relief, reduce postoperative ileus, enhance bowel recovery and minimalize risk of cardiopulmonary complications. Literature suggests usage of additional procedures such as locoregional blocks and continuous infusion of local anaesthetics via pre-peritoneal wound catheters, however, the level of evidence is very low. Additionally, Alvimopan administration is suggested. Alvimopan is a peripherally acting µ-opioid receptor with limited ability to cross the blood-brain barrier, which therefore reduces many of the undesirable opioid side effects without affecting analgesia (39-41).
Evidence for RC/grade of recommendation: not available/strong.
Early mobilization
Prolonged bed rest increases postoperative complications risk such as thromboembolism and pulmonary events. Therefore, early mobilization is recommended.
Evidence for RC/grade of recommendation: not available/strong.
Early oral diet
It has been shown that early enteral feeding is not related to an elevated risk of bowel anastomosis leak/dehiscence, however, it is associated with a higher risk of nausea and vomiting, especially when opiates are used. With early enteral nutrition, the proper nitrogen compounds balance metabolism is preserved and the tissue insulin resistance is reduced. In recent study comparing early oral feeding with nasojejunal tube feeding after RC have shown that oral feeding is safe and well tolerated, yet, the rate of postoperative ileus was significantly lower in nasojejunal tube group. Another study has proven that total parenteral nutrition after RC is associated with a higher incidence of complications, mainly infections, and higher costs (17,42-44).
Evidence for RC/grade of recommendation: not available/strong.
Audit
Audits are generally aimed to improve quality of care by assessing both clinical and non-clinical outcomes, measuring ERAS protocol compliance and finally maintaining the concept vivid.
Evidence for RC/grade of recommendation: not available/strong.
ERAS in RC setting
After publication of ERAS RC guidelines various authors tried to assess and evaluate its usefulness and effectiveness. Some observational papers proved that enhanced recovery programs are feasible, safe, and at least not inferior to conventional treatment protocols (45-47). Later, numerous papers compering the two approaches were published. Different studies compared various factors, with most commonly assessing LoS, complications, and some form of bowel recovery (Table 1). Brief description of the studies is presented below.
Table 1
| First author | Year | Country | Design | No. of patients | Operation type (ERAS*/non-ERAS) | Main findings for ERAS* group | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ERAS* | non-ERAS | ORC | LRC | RARC | ||||||
| Saar (48) | 2013 | Germany | Prospective | 31 | 31 | – | – | 31/31 | Earlier patient mobilisation, faster return to a regular diet and lower analgesics requirements | |
| Mukhtar (49) | 2013 | UK | Prospective | 51 | 26 | 51/26 | – | – | Shorter LoS and intensive care unit stay. Shorter time to nasogastric tube removal, time to passage of flatus and faeces and faster return to a regular diet | |
| Karl (50) | 2014 | Germany | Prospective | 62 | 39 | – | – | – | Lower incidence of fever and deep venous thrombosis, lower analgesics requirements, higher (better) QoL scores | |
| Frees (51) | 2018 | Canada | Prospective | 10 | 13 | – | – | – | Shorter LoS, shorter time to first bowel movement, higher nausea levels | |
| Pang (52) | 2017 | UK | Prospective | 393 | 60 | – | – | 425/28 | Shorter LoS, lower blood loss, lower transfusion rate, fewer readmissions | |
| Lin (53) | 2018 | China | Prospective | 144 | 145 | 25/34 | 112/103 | 7//8 | Shorter time to first bowel movement, faster fluid and regular diet tolerance, earlier patient mobilisation | |
| Semerjian (54) | 2018 | USA | Prospective | 56 | 54 | 48/52 | – | 8//2 | Shorter LoS, lower median hospital charge | |
| Palumbo (55) | 2018 | Italy | Prospective | 74 | 40 | 74/40 | – | – | Lower incidence of 90-day complications, faster bowel recovery | |
| Smith (56) | 2014 | UK | Retrospective | 37/27 | 69 | 37/27/69 | – | – | Shorter LoS, lower rate of ileus | |
| Cerruto (57) | 2014 | Italy | Retrospective | 9 | 13 | – | – | – | Shorter time to flatus | |
| Guan (58) | 2014 | China | Retrospective | 60 | 55 | – | 60/55 | – | Shorter LoS, shorter time to flatus, lower incidence of complications, lower mean levels of WBC and CRP | |
| Persson (59) | 2015 | Sweden | Retrospective | 31 | 39 | 31/39 | – | – | Shorter time to first stool, lower readmission rate | |
| Koupparis (60) | 2015 | UK | Retrospective | 56 | 56 | – | – | – | Shorter LoS, lower transfusion rate | |
| Xu (61) | 2015 | USA | Retrospective | 124 | 81 | 124/81 | – | – | Shorter LoS, lower analgesics requirements, more pain after surgery, lower rate of ileus | |
| Collins (62) | 2016 | Sweden | Retrospective | 135 | 86 | – | – | 135/86 | Shorter LoS | |
| Wei (63) | 2018 | China | Retrospective | 91 | 101 | – | – | – | Shorter times to liquid intake, first ambulation, flatus, first defecation and pelvic drainage tube removal. Lower blood loss, lower rates of transfusions, readmissions, and complications | |
| Bazargani (64) | 2018 | USA | Retrospective | 145 | 144 | 145/144 | – | – | Shorter LoS, shorter time to flatus, lower incidence of gastrointestinal complications | |
| Kukreja (65) | 2018 | USA | Retrospective | 245 | 138 | 164/108 | – | 81/30 | Less severe sensation of dry mouth, disturbed sleep, drowsiness, and pain | |
| Dunkman (66) | 2019 | USA | Retrospective | 100 | 100 | – | – | – | Shorter LoS, lower blood loss, lower transfusion rate, lower readmission rate, shorter time to first stool | |
| Zhang (67) | 2019 | China | Retrospective | 185 | 258 | – | ERAS group | – | Shorter LoS, lower blood loss, lower transfusion rate, shorter times to tolerate a liquid diet, first ambulation and first flatus. lower incidence of complications. Shorter time to pelvic drainage removal, lower readmission rate | |
| Baack Kukreja (68) | 2017 | USA | Retrospective | 79 | 121 | 92 | – | 108 | Shorter LoS, lower incidence of myocardial infarction, ileus, requirement for total parenteral nutrition, and ventilator support for >48 h | |
*, patients included in the studies are treated according ERAS guidelines, yet, not in all studies all particular ERAS details are followed. ERAS, enhanced recovery after surgery; ORC, open radical cystectomy; LRC, laparoscopic radical cystectomy; RARC, robot-assisted radical cystectomy; LoS, length of stay; QoL, quality of life; WBC, white blood cells; CRP, C-reactive protein.
Prospective studies
Saar et al. (48) conducted a non-randomized study on 62 patients operated laparoscopically (31 patients treated conventionally vs. 31 patients with fast-track). They showed that fast-track protocol resulted in earlier patient mobilisation, faster return to a regular diet and lower analgesics requirements, as well as, earlier (yet, not statistically significant) return of bowel function. There were no differences in absolute, nor high grade complications number between two groups.
In other paper, Mukhtar et al. (49) included 26 consecutive patients (open RC) treated conventionally and 51 patients within an enhanced recovery programme (ERP). The median LoS was shortened for almost 1 day and the mean intensive care unit stay was shortened for more than 1 day for ERP. Time to nasogastric tube removal, time to passage of flatus and faeces and time to full diet were statistically shorter in the ERP population. Clavien-Dindo complications and mortality rates were comparable in both study arms.
Karl et al. (50) in a study on 101 patients analysed QoL using a validated questionnaire (EORTC QLQ-30). They proved that at the time of discharge, in most subgroups of the questionnaire, the ERAS group fared statistically better than the conventional arm. Additionally, morbidity after operation was lower in the ERAS population in terms of wound healing, fever and deep venous thrombosis. Also, the demand for analgesic was statistically lower in the ERAS population.
In the next trial by Frees et al. (51) on 23 patients, authors demonstrated that LoS was more than 1 day shorter and time to first bowel movement was 2 days shorter in ERAS group. However, higher nausea levels were noted. There were no differences in complications rates as well as in mental wellbeing and QoL levels measured by psychological questioners.
Pang et al. (52) proved in the analysis of 453 consecutive patients (87% with ERAS) that LoS was shorter for ERAS. Additionally, patients with ERAS had lower blood loss, lower transfusion rates and fewer readmissions.
In the other randomized, multicentric trial by Lin et al. (53) authors assessed 290 patients (145 ERAS vs. 145 conventional) and showed that there were no differences in complications rate LoS period and time to flatus between the study arms. On the other hand, the return of bowel movement, fluid diet tolerance, regular diet tolerance, and ambulation were significantly faster in the ERAS group.
Semerjian et al. (54) conducted economic analysis assessing 110 patients (56 ERAS and 54 historical controls) and proved that median charge for index hospitalization was almost $5,000 lower for ERAS patients. Those differences arose mainly from shorter LoS. The overall complication and readmission rates were similar between the two groups.
In the last prospective study by Palumbo et al. (55) (74 ERAS vs. 40 controls) it was shown that bowel function recovery was significantly faster in the ERAS group. Bowel sounds we recorded on postoperative day 1 in 58% vs. 10% patients, passage of flatus within postoperative day 2 in 55% vs. 28% patients, and passage of stool within postoperative day 3 in 50% vs. no patients in the ERAS vs. control group. Also 90-day complications were higher in the control group.
Retrospective studies
In the analysis of 133 consecutive patients by Smith et al. (56) authors compared three consecutive cohorts of patients: (I) no-ERP, (II) primary stage ERP patients and (III) latter stage ERP patients. Patients underwent ileal conduit operation, yet, some of them did not received lymphadenectomy. Authors showed successive reductions in the observed rates of ileus and a significant improvement in LoS in later cohorts.
Further, in the “before and after” analysis by Cerruto et al. (57) on 22 patients, the time of flatus was statistically earlier in the ERP population, as was the time to start a light diet.
In the “before and after” study by Guan et al. (58) 55 patients underwent laparoscopic RC before and 60 patients after fast-track program introduction. There were statistically more complications in the conventional group, yet, majority of them were minor. What is more, fast-track patients were discharged from hospital earlier and duration time to first flatus, time to regular diet were shorter and hospital expenses were statistically lower. Interestingly, authors compared white blood cell (WBC) counts and the levels of serum C-reactive protein (CRP), which reflect the surgical stress response. In postoperative days 5 and 7, the mean levels of WBC and CRP in the fast-track group were significantly lower than the conventional group.
In another analysis by Persson et al. (59) including 31 ERAS patients and 39 controls authors demonstrated that the ERAS group had statistically shorter mean time to first stool passage and lower readmission frequency than the controls. No differences in high grade complications or LoS were noted.
Koupparis et al. (60) compared 102 consecutive patients undergoing robotic-assisted RC (RARC) with historical open cohort (ORC). A significant reduction in transfusion rate was seen in robotic arm. Also, there was non-statistically significant trend to a lower total complications rate in robotic group. The median LoS, likewise, was shorter for the RARC group and the mortality rates were equivalent between the groups.
In the study by Xu et al. (61) analysing mainly pain management after RC authors showed that patients on enhanced recovery used significantly less opioids per day, yet, reported more pain after surgery. Also, incidence of postoperative ileus was lower and LoS was shorter in ERAS group.
Collins et al. (62) demonstrated in the analysis on 221 patients that LoS was shorter for ERAS group. Readmission rate and overall as well as high-grade complication rates did not differ significantly between the groups. However, it has to be highlighted that no epidural anaesthesia was used in the ERAS group.
Wei et al. (63) presented a study on 192 patients (91 ERAS vs. 101 conventional) and showed that the times to liquid intake, first ambulation, flatus, first defecation, and pelvic drainage tube removal were significantly shorter in ERAS group. The intraoperative blood loss volume, blood transfusion rate, readmission rate, and incidence of postoperative complications were also significantly lower in the ERAS group.
In the next “before and after” analysis by Bazargani et al. (64), 145 patients with ERAS operated openly were matched to 144 historical controls. Authors determined that the time from surgery to first flatus and LoS were statistically shorter in ERAS group. There was no significant difference in minor, major, or overall complications or readmission rates between the two groups, yet, the rate of 30-day gastrointestinal complications was significantly lower in the ERAS cohort.
Kukreja et al. (65) conducted a study assessing patient-reported outcomes (PRO) with the MD Anderson Symptom Inventory (MDASI)—a validated tool for capturing multiple PROs, including pain, among patients with cancer. From total 383 cases, 245 patients were being treated on an ERAS pathway and 138 were being treated on a traditional-care pathway. Authors showed that dry mouth, disturbed sleep, drowsiness, and pain were significantly less-severe for patients in ERAS group.
The recent study by Dunkman et al. (66) was an analysis of 200 patients. Authors showed that in the ERAS group there was decrease in estimated intraoperative blood loss with corresponding decrease in intraoperative packed red blood cell and intraoperative fresh frozen plasma transfusions. Also, the median LoS decreased from 10 to 7 days and readmission rate was lower. There were significant reductions in time to first stool, to self-stoma management, and in opioid usage. Overall complication rates were lower or unchanged in the ERAS group compared to the historical control group.
In the paper by Zhang et al. (67) on 443 patients (185 ERAS vs. 258 conventional), ERAS group presented lower intraoperative blood loss and transfusion rates. What is more, patients in the ERAS population had shorter times to tolerate a liquid diet, first ambulation and first flatus. The risk of complications was also significantly lower in the ERAS arm. The time to pelvic drainage removal, readmission rate and LoS were significantly shorter in the ERAS population.
Baack Kukreja et al. (68) presented paper assessing cystectomy enhanced recovery pathway (CERP). It varies from ERAS pathway by not using home i.v. fluids but administering a gentle bowel regimen. After the propensity matching analysis, it was demonstrated that general complications did not differ between study groups, however, postoperative myocardial infarction, ileus, requirement for total parenteral nutrition, and ventilator support for >48 hours were higher in control group. Also, there were no differences between the two groups in the number of readmissions and emergency department visits. The LOS was significantly different and shorter for CERP group.
Metanalyses
Up to date three metanalyses assessing ERAS protocol in RC setting were published. First one included studies published up to February 2016. Thirteen studies included 1,493 patients in total (ERAS: 801, standard care: 692), yet, only 3 of those papers were purely prospective. Authors demonstrated that ERAS did not reduce the readmission and mortality rate, however, the complication risk was lower in ERAS group (mostly low-grade complications). The number needed to treat to prevent one complication was approximately 14. What is more, LoS was shorter in ERAS group with mean difference between groups of approximately 5.4 days. Time to return of bowel was also faster in ERAS population—the estimated mean difference was 1.1 d in favour of ERAS (69). Second metanalysis was published by Xiao et al. and included 1,258 ERAS cases and 842 cases treated conventionally. Authors proved that the time to first flatus passage, time until return to a regular diet and the LoS were significantly shorter when ERAS was used. Moreover, the incidence of postoperative complications (especially postoperative paralytic ileus) and cardiovascular events were significantly lower in the ERAS group (70). Third paper included 27 studies, with 3 randomized and 24 non-randomized controlled studies. A total of 4,712 patients were analysed—2,690 (57%) participants with ERAS protocol and 2,022 (43%) controls receiving standard of care. Authors demonstrated that ERAS protocols were associated with faster recovery of bowel function, earlier return to solid diet and shorter LoS. Additionally, 30-day and 90-day major complication, mortality or readmission rates did not differ significantly (71).
Discussion
ERAS program is an important step forward in the setting of extremely morbid RC. Emerging studies show almost unanimously that implementation of ERAS significantly improves RC outcomes (mainly LoS and bowel recovery) and the final treatment cost. Nevertheless, some blemishes of available papers must be discussed. The biggest ERAS demerit is the fact, that because of lack of solid data in RC setting, some ERAS recommendations were extrapolated from gastro-intestinal surgery. With time passing by, the amount of evidence is growing, yet, the available studies are very difficult to compare. Therefore, the universal conclusions and recommendations are hard to be drawn. The biggest limitation of the available literature is the heterogeneity of the studies considering patient population, surgical variables (surgical approach, urinary diversion modality, length of bowel used, ureteral anastomosis stenting, range of lymphadenectomy), and the ERAS and standard of care protocols. Each study uses slightly distinct protocols from all pathways used in the other articles. Additionally, compliance with all ERAS protocols in large studies reporting on colorectal surgery is generally not higher than 60% when considering the personnel compliance. In case of patient compliance, it is probably even lower. What is more, the possible influence of neoadjuvant chemotherapy is rarely discussed in the papers. Some irregularities may be also due to the fact that studies are conducted in different healthcare economic systems with variable procedures coverage criteria. Moreover, a lot of studies compare consecutive periods of time before and after ERAS implementation. In some papers, authors exclude 20–30 cases that were performed in the “transitional” period, however, some bias may be present because of “learning” curve phenomenon. Finally, numerous definitions of various clinical situations (e.g., ileus, flatus) and complications reporting systems are used in available papers.
Conclusions
RC is associated with severe morbidity. It is highly recommended to follow evidence-based fast-track/ERAS guidelines to lower the risk of complications and optimize the results. However, without prospective, randomized, big population studies analysing separately each ERAS point in RC, we will not be sure which particular ERAS element is beneficial and which is neutral or even detrimental.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Marco Moschini) for the series “Bladder Cancer” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj.2019.12.01/coif). The series “Bladder Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017;71:96-108. [Crossref] [PubMed]
- Babjuk M, Bohle A, Burger M, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Cerantola Y, Valerio M, Persson B, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS((R))) society recommendations. Clin Nutr 2013;32:879-87. [Crossref] [PubMed]
- Lawrentschuk N, Colombo R, Hakenberg OW, et al. Prevention and management of complications following radical cystectomy for bladder cancer. Eur Urol 2010;57:983-1001. [Crossref] [PubMed]
- Konety BR, Allareddy V, Herr H. Complications after radical cystectomy: analysis of population-based data. Urology 2006;68:58-64. [Crossref] [PubMed]
- Kim SP, Shah ND, Karnes RJ, et al. The implications of hospital acquired adverse events on mortality, length of stay and costs for patients undergoing radical cystectomy for bladder cancer. J Urol 2012;187:2011-7. [Crossref] [PubMed]
- Kim SP, Boorjian SA, Shah ND, et al. Contemporary trends of in-hospital complications and mortality for radical cystectomy. BJU Int 2012;110:1163-8. [Crossref] [PubMed]
- Hautmann RE, Hautmann SH, Hautmann O. Complications associated with urinary diversion. Nat Rev Urol 2011;8:667-77. [Crossref] [PubMed]
- Albisinni S, Rassweiler J, Abbou CC, et al. Long-term analysis of oncological outcomes after laparoscopic radical cystectomy in Europe: results from a multicentre study by the European Association of Urology (EAU) section of Uro-technology. BJU Int 2015;115:937-45. [Crossref] [PubMed]
- van Hemelrijck M, Thorstenson A, Smith P, et al. Risk of in-hospital complications after radical cystectomy for urinary bladder carcinoma: population-based follow-up study of 7608 patients. BJU Int 2013;112:1113-20. [Crossref] [PubMed]
- Dybowski B, Ossolinski K, Ossolinska A, et al. Impact of stage and comorbidities on five-year survival after radical cystectomy in Poland: single centre experience. Cent European J Urol 2015;68:278-83. [Crossref] [PubMed]
- Masago T, Morizane S, Honda M, et al. Estimation of mortality and morbidity risk of radical cystectomy using POSSUM and the Portsmouth predictor equation. Cent European J Urol 2015;68:270-6. [Crossref] [PubMed]
- Yuh BE, Nazmy M, Ruel NH, et al. Standardized analysis of frequency and severity of complications after robot-assisted radical cystectomy. Eur Urol 2012;62:806-13. [Crossref] [PubMed]
- Djaladat H, Daneshmand S. Gastrointestinal Complications in Patients Who Undergo Radical Cystectomy with Enhanced Recovery Protocol. Curr Urol Rep 2016;17:50. [Crossref] [PubMed]
- Nielsen ME, Mallin K, Weaver MA, et al. Association of hospital volume with conditional 90-day mortality after cystectomy: an analysis of the National Cancer Data Base. BJU Int 2014;114:46-55. [Crossref] [PubMed]
- Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009;55:164-74. [Crossref] [PubMed]
- Krajewski W, Zdrojowy R, Tupikowski K, et al. How to lower postoperative complications after radical cystectomy - a review. Cent European J Urol 2016;69:370-6. [PubMed]
- Krajewski W, Piszczek R, Krajewska M, et al. Urinary diversion metabolic complications - underestimated problem. Adv Clin Exp Med 2014;23:633-8. [Crossref] [PubMed]
- Hijazi Y, Gondal U, Aziz O. A systematic review of prehabilitation programs in abdominal cancer surgery. Int J Surg 2017;39:156-62. [Crossref] [PubMed]
- Minnella EM, Awasthi R, Bousquet-Dion G, et al. Multimodal Prehabilitation to Enhance Functional Capacity Following Radical Cystectomy: A Randomized Controlled Trial. Eur Urol Focus 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Oberle AD, West JM, Tobert CM, et al. Optimizing Nutrition Prior to Radical Cystectomy. Curr Urol Rep 2018;19:99. [Crossref] [PubMed]
- Muñoz M, Acheson AG, Auerbach M, et al. International consensus statement on the peri-operative management of anaemia and iron deficiency. Anaesthesia 2017;72:233-47. [Crossref] [PubMed]
- Ritch CR, Cookson MS, Clark PE, et al. Perioperative Oral Nutrition Supplementation Reduces Prevalence of Sarcopenia following Radical Cystectomy: Results of a Prospective Randomized Controlled Trial. J Urol 2019;201:470-7. [Crossref] [PubMed]
- Burden S, Billson HA, Lal S, et al. Perioperative nutrition for the treatment of bladder cancer by radical cystectomy. Cochrane Database Syst Rev 2019;5:CD010127. [PubMed]
- Cui HW, Turney BW, Griffiths J. The Preoperative Assessment and Optimization of Patients Undergoing Major Urological Surgery. Curr Urol Rep 2017;18:54. [Crossref] [PubMed]
- Moran J, Wilson F, Guinan E, et al. Role of cardiopulmonary exercise testing as a risk-assessment method in patients undergoing intra-abdominal surgery: a systematic review. Br J Anaesth 2016;116:177-91. [Crossref] [PubMed]
- Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489-95. [Crossref] [PubMed]
- Xu R, Zhao X, Zhong Z, et al. No advantage is gained by preoperative bowel preparation in radical cystectomy and ileal conduit: a randomized controlled trial of 86 patients. Int Urol Nephrol 2010;42:947-50. [Crossref] [PubMed]
- Tan WS, Tan MY, Lamb BW, et al. Intracorporeal robot-assisted radical cystectomy, together with an enhanced recovery programme, improves postoperative outcomes by aggregating marginal gains. BJU Int 2018;121:632-9. [Crossref] [PubMed]
- Novara G, Catto JW, Wilson T, et al. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur Urol 2015;67:376-401. [Crossref] [PubMed]
- Wuethrich PY, Burkhard FC, Thalmann GN, et al. Restrictive deferred hydration combined with preemptive norepinephrine infusion during radical cystectomy reduces postoperative complications and hospitalization time: a randomized clinical trial. Anesthesiology 2014;120:365-77. [Crossref] [PubMed]
- Wuethrich PY, Studer UE, Thalmann GN, et al. Intraoperative continuous norepinephrine infusion combined with restrictive deferred hydration significantly reduces the need for blood transfusion in patients undergoing open radical cystectomy: results of a prospective randomised trial. Eur Urol 2014;66:352-60. [Crossref] [PubMed]
- Burkhard FC, Studer UE, Wuethrich PY. Superior functional outcome after radical cystectomy and orthotopic bladder substitution with restrictive intraoperative fluid management: a followup study of a randomized clinical trial. J Urol 2015;193:173-8. [Crossref] [PubMed]
- Myrga JM, Ayyash OM, Bandari J, et al. The Safety and Short-term Outcomes of Leukocyte Depleted Autologous Transfusions During Radical Cystectomy. Urology 2020;135:106-10. [PubMed]
- Myles PS, Bellomo R, Corcoran T, et al. Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N Engl J Med 2018;378:2263-74. [Crossref] [PubMed]
- Bazargani ST, Ghodoussipour S, Tse B, et al. The association between intraoperative fluid intake and postoperative complications in patients undergoing radical cystectomy with an enhanced recovery protocol. World J Urol 2018;36:401-7. [Crossref] [PubMed]
- Ziouziou I, Ammani A, Karmouni T, et al. Does chewing gum improve postoperative results in patients undergoing radical cystectomy? A systematic review of literature and meta-analysis. Prog Urol 2017;27:513-20. [Crossref] [PubMed]
- Pruthi RS, Nielsen M, Smith A, et al. Fast track program in patients undergoing radical cystectomy: results in 362 consecutive patients. J Am Coll Surg 2010;210:93-9. [Crossref] [PubMed]
- Lee CT, Chang SS, Kamat AM, et al. Alvimopan accelerates gastrointestinal recovery after radical cystectomy: a multicenter randomized placebo-controlled trial. Eur Urol 2014;66:265-72. [Crossref] [PubMed]
- Cui Y, Chen H, Qi L, et al. Effect of alvimopan on accelerates gastrointestinal recovery after radical cystectomy: A systematic review and meta-analysis. Int J Surg 2016;25:1-6. [Crossref] [PubMed]
- Sultan S, Coles B, Dahm P. Alvimopan for recovery of bowel function after radical cystectomy. Cochrane Database Syst Rev 2017;5:CD012111. [PubMed]
- Roth B, Birkhauser FD, Zehnder P, et al. Parenteral nutrition does not improve postoperative recovery from radical cystectomy: results of a prospective randomised trial. Eur Urol 2013;63:475-82. [Crossref] [PubMed]
- Voskuilen CS, van de Putte EEF, der Hulst JB, et al. Short-term outcome after cystectomy: comparison of early oral feeding in an enhanced recovery protocol and feeding using Bengmark nasojejunal tube. World J Urol 2018;36:221-9. [Crossref] [PubMed]
- Deibert CM, Silva MV. A Prospective Randomized Trial of the Effects of Early Enteral Feeding After Radical Cystectomy. Urology 2016;96:69-73. [Crossref] [PubMed]
- Dutton TJ, Daugherty MO, Mason RG, et al. Implementation of the Exeter enhanced recovery programme for patients undergoing radical cystectomy. BJU Int 2014;113:719-25. [Crossref] [PubMed]
- Daneshmand S, Ahmadi H, Schuckman AK, et al. Enhanced recovery protocol after radical cystectomy for bladder cancer. J Urol 2014;192:50-5. [Crossref] [PubMed]
- Miller C, Campain NJ, Dbeis R, et al. Introduction of robot-assisted radical cystectomy within an established enhanced recovery programme. BJU Int 2017;120:265-72. [Crossref] [PubMed]
- Saar M, Ohlmann CH, Siemer S, et al. Fast-track rehabilitation after robot-assisted laparoscopic cystectomy accelerates postoperative recovery. BJU Int 2013;112:E99-106. [Crossref] [PubMed]
- Mukhtar S, Ayres BE, Issa R, et al. Challenging boundaries: an enhanced recovery programme for radical cystectomy. Ann R Coll Surg Engl 2013;95:200-6. [Crossref] [PubMed]
- Karl A, Buchner A, Becker A, et al. A new concept for early recovery after surgery for patients undergoing radical cystectomy for bladder cancer: results of a prospective randomized study. J Urol 2014;191:335-40. [Crossref] [PubMed]
- Frees SK, Aning J, Black P, et al. A prospective randomized pilot study evaluating an ERAS protocol versus a standard protocol for patients treated with radical cystectomy and urinary diversion for bladder cancer. World J Urol 2018;36:215-20. [Crossref] [PubMed]
- Pang KH, Groves R, Venugopal S, et al. Prospective Implementation of Enhanced Recovery After Surgery Protocols to Radical Cystectomy. Eur Urol 2018;73:363-71. [PubMed]
- Lin T, Li K, Liu H, et al. Enhanced recovery after surgery for radical cystectomy with ileal urinary diversion: a multi-institutional, randomized, controlled trial from the Chinese bladder cancer consortium. World J Urol 2018;36:41-50. [Crossref] [PubMed]
- Semerjian A, Milbar N, Kates M, et al. Hospital Charges and Length of Stay Following Radical Cystectomy in the Enhanced Recovery After Surgery Era. Urology 2018;111:86-91. [Crossref] [PubMed]
- Palumbo V, Giannarini G, Crestani A, et al. Enhanced Recovery After Surgery Pathway in Patients Undergoing Open Radical Cystectomy Is Safe and Accelerates Bowel Function Recovery. Urology 2018;115:125-32. [Crossref] [PubMed]
- Smith J, Meng ZW, Lockyer R, et al. Evolution of the Southampton Enhanced Recovery Programme for radical cystectomy and the aggregation of marginal gains. BJU Int 2014;114:375-83. [PubMed]
- Cerruto MA, De Marco V, D'Elia C, et al. Fast track surgery to reduce short-term complications following radical cystectomy and intestinal urinary diversion with Vescica Ileale Padovana neobladder: proposal for a tailored enhanced recovery protocol and preliminary report from a pilot study. Urol Int 2014;92:41-9. [Crossref] [PubMed]
- Guan X, Liu L, Lei X, et al. A comparative study of fast-track versus [corrected] conventional surgery in patients undergoing laparoscopic radical cystectomy and ileal conduit diversion: Chinese experience. Sci Rep 2014;4:6820. [Crossref] [PubMed]
- Persson B, Carringer M, Andren O, et al. Initial experiences with the enhanced recovery after surgery (ERAS) protocol in open radical cystectomy. Scand J Urol 2015;49:302-7. [Crossref] [PubMed]
- Koupparis A, Villeda-Sandoval C, Weale N, et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion: impact on an established enhanced recovery protocol. BJU Int 2015;116:924-31. [Crossref] [PubMed]
- Xu W, Daneshmand S, Bazargani ST, et al. Postoperative Pain Management after Radical Cystectomy: Comparing Traditional versus Enhanced Recovery Protocol Pathway. J Urol 2015;194:1209-13. [Crossref] [PubMed]
- Collins JW, Adding C, Hosseini A, et al. Introducing an enhanced recovery programme to an established totally intracorporeal robot-assisted radical cystectomy service. Scand J Urol 2016;50:39-46. [Crossref] [PubMed]
- Wei C, Wan F, Zhao H, et al. Application of enhanced recovery after surgery in patients undergoing radical cystectomy. J Int Med Res 2018;46:5011-8. [Crossref] [PubMed]
- Bazargani ST, Djaladat H, Ahmadi H, et al. Gastrointestinal Complications Following Radical Cystectomy Using Enhanced Recovery Protocol. Eur Urol Focus 2018;4:889-94. [Crossref] [PubMed]
- Kukreja JB, Shi Q, Chang CM, et al. Patient-Reported Outcomes Are Associated With Enhanced Recovery Status in Patients With Bladder Cancer Undergoing Radical Cystectomy. Surg Innov 2018;25:242-50. [Crossref] [PubMed]
- Dunkman WJ, Manning MW, Whittle J, et al. Impact of an enhanced recovery pathway on length of stay and complications in elective radical cystectomy: a before and after cohort study. Perioper Med (Lond) 2019;8:9. [Crossref] [PubMed]
- Zhang H, Wang H, Zhu M, et al. Implementation of enhanced recovery after surgery in patients undergoing radical cystectomy: A retrospective cohort study. Eur J Surg Oncol 2020;46:202-8. [PubMed]
- Baack Kukreja JE, Kiernan M, Schempp B, et al. Quality Improvement in Cystectomy Care with Enhanced Recovery (QUICCER) study. BJU Int 2017;119:38-49. [Crossref] [PubMed]
- Tyson MD, Chang SS. Enhanced Recovery Pathways Versus Standard Care After Cystectomy: A Meta-analysis of the Effect on Perioperative Outcomes. Eur Urol 2016;70:995-1003. [Crossref] [PubMed]
- Xiao J, Wang M, He W, et al. Does Postoperative Rehabilitation for Radical Cystectomy Call for Enhanced Recovery after Surgery? A Systematic Review and Meta-analysis. Curr Med Sci 2019;39:99-110. [Crossref] [PubMed]
- Giannarini G, Crestani A, Inferrera A, et al. Impact of enhanced recovery after surgery protocols versus standard of care on perioperative outcomes of radical cystectomy: a systematic review and meta-analysis of comparative studies. Minerva Urol Nefrol 2019;71:309-23. [Crossref] [PubMed]
Cite this article as: Krajewski W, Zdrojowy R. Enhanced recovery after surgery (ERAS) in patients treated with radical cystectomy. AME Med J 2020;5:3.

