
Review on urethral cancer: what do you need to know
Introduction
Primary urethral cancer (PUC) is a rare malignancy accounting for less than 1% of all malignancies (1) with an incidence rate of 4.3 per million men and 1.5 per million women in the United States annually (2). The three major histological subtypes of PUC are urothelial carcinoma (UC), squamous cell carcinoma (SCC), and adenocarcinoma (AC) (3). Current standards for treating PUC are not well established, and there is a high demand for a consensus on the best treatment strategy for treating PUC (4). Due to the rarity of the disease, most current studies are retrospective with small sample sizes and inconsistent patient demographics; therefore, it is difficult to generalize about the best treatment regimen for this rare disease (5). There is a wide variety of literature topics on PUC, but this review focuses on critically analyzing the various treatment options and prognosis in an attempt to determine the best treatment modality for treating PUC.
Diagnosis and imaging
The American Joint Committee on Cancer developed a TNM staging system specifically for PUC (Table 1) (6,7). The T is determined by the extent of tumor invasion, N is extent of lymph node involvement, and M is a measurement of the extent of metastasis. This TNM system is set up in order to quantify the extent of PUC involvement in a patient to be used as an algorithm key for developing a treatment strategy. The diagnosis and staging of PUC are determined by a combination of physical exam, imaging, and biopsy (Figure 1) (8).
Table 1
| Stages | Details |
|---|---|
| T stage | |
| Tx | Tumor cannot be assessed |
| Tis | Carcinoma in situ |
| Ta | Noninvasive carcinoma |
| T1 | Lamina propria invasion |
| T2 | Spongiosum, prostate, or periurethral muscle invasion |
| T3 | Cavernosum, vagina, or bladder neck invasion |
| T4 | All regional nodes are negative |
| N stage | |
| N0 | All regional nodes are negative |
| N1 | Single positive node <2 cm |
| N2 | Single positive node >2 cm or multiple nodes |
| M stage | |
| M0 | No metastasis |
| M1 | Distant metastasis |
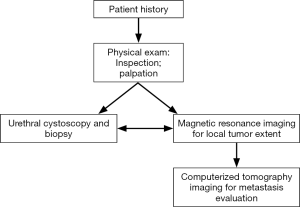
PUC is very rare and difficult to detect, so this tumor is often not detected until later stages (9). Current methods for detecting PUC involve physical exam, cystoscopy with confirmatory tissue biopsy, and imaging. The elusiveness and rarity of this malignancy may make it overlooked until later stages—delaying treatment until it manifests into advanced disease thus reducing overall survival. Since there are more positive responses with early stage and asymptomatic PUC (9-11), it is optimal to detect these tumors early before symptoms arise.
Touijer and Dalbagni (12) studied the effectiveness of voided urine cytology for detecting PUC. They found that the highest sensitivity was with the detection of SCC at 77% and lowest for UC at 50%. They concluded that voided urine cytologies are not effective for diagnosing PUC in both males and females. A cystourethroscopy with biopsy is the gold standard which should be done if there is suspicion of PUC.
Both T1- and T2-weighted magnetic resonance imaging (MRI) is used for determining staging and evaluating the local extent of PUC involvement (8,13). Imaging may be more beneficial for detecting proximal tumors where physical exam cannot detect (13). These tumors show decreased signal intensity when compared to surrounding tissue in both T1 and T2 weighted images. Additionally, tumors extending into the tunica albuginea or corpus cavernosum can be seen on T2-weighted MRI easily in males (13). MRI has a detection accuracy of 90% in females (14) and an overall detection accuracy of 75% (8). Despite the accuracy of MRI, malignant and benign tumors cannot be differentiated in this imaging, so biopsy is still necessary for proper diagnosis (8).
Gourtsoyianni and colleagues (15) noted that MRI following chemoradiotherapy can determine the extent of disease and nodal status, but this study has limitations through its very small sample size and retrospective analysis. Despite these limitations, this study shows that MRI has additional benefits in treatment monitoring in addition to diagnosis.
Surgery and radiation therapy
Male PUC
The goal of treating male PUC with surgery is to resect the tumor with a tumor clearance margin of 5 mm while preserving the function of the penis (16,17). Common surgical procedures for treating PUC include all of the following: partial/total penectomy, urethrectomy, and transurethral resection (the preferred option being best suited by the location, size, and depth of the lesion); however, penile preserving surgeries should be considered when possible as they may result in similar oncological outcomes while improving psychological outcomes (16,18). Distal (anterior) tumors in males are limited to the penile urethra and often are of SCC histology (19). These tumors are often more easily detected and offer better prognosis (13). Proximal (posterior) tumors in males are limited to the bulbous urethra and extend to the prostatic urethra and are often of histological subtypes SCC, AC, and UC (19). Proximal tumors tend to have worse prognosis than distal tumors (11,19).
Men often present with UC type tumors which tend to reside in the proximal urethra (19,20). Proximal tumors are difficult to treat since they often present with little to no symptoms and are thus detected at later stages (11,13,19,21). At later stages, tumor spread may occur to nearby structures (i.e., prostate, bone, and lymph nodes) and therefore may require a multidisciplinary approach consisting of urologists, radiologists, radiation oncologists, oncologists, and orthopedics (3). Proximal tumors are often treated surgically with a total penectomy and cystoprostatectomy, but efforts are being made to move towards penile-preserving surgery (3,18). In addition, reconstructive efforts can be made towards patients undergoing a total penectomy such as a phalloplasty utilizing flaps (such as radial artery free flap phalloplasty used 90% of the time) with or without penile prosthesis with advancements being made towards stem cell phallic regeneration and transplantation (16).
A study by Rabbani (22) evaluated whether radiation in combination with surgery has better outcomes for treating PUC. In this study, patients with AC histology of the proximal urethra showed better outcomes with radiation (external beam and/or brachytherapy) alone compared to surgery or combination therapy (i.e., surgery combined with radiation). One concern when treating a patient with proximal tumors is prostatic involvement. A retrospective study of 1,506 patients with UC revealed prostate involvement with UC is significantly associated with the risk of urethral recurrence following radical cystectomy (P<0.0001) (11); however, it is not clear in this study whether this urethral involvement is due to PUC or urethral carcinoma second to bladder cancer.
Distal PUC in men is often of type SCC and offers better prognosis (13,21). Distal PUC is often treated surgically with local excision or partial/total penectomy, and distal PUC has more potential for penile preserving surgeries such as hypospadia formation, urethroplasty, or glansectomy with grafting than proximal tumors (3,18). Although distal tumors are easier to detect and often present as earlier stages than their proximal counterparts, lymph node involvement should always be assessed (3). Werntz and colleagues (23) reviewed the effects of inguinal lymph node dissection in men with SCC of the distal urethra. They found that lymph node positivity was associated with worse overall survival, and inguinal lymph node dissection improves overall survival in patients with N1 or N2 disease. They report that PUC (T1-T4) has node involvement in 9% of cases, and prophylactic inguinal lymph node dissection should not be considered for N0 disease.
Radiation therapy may be considered in addition to surgery for treating distal PUC (3). Eng and colleagues (10) showed that surgery alone was often the preferred treatment for early stage SCC; however, Rabbani (22) noticed that there is no significant difference in outcomes between surgery alone and radiation alone for treating SCC. This information may be due to the fact that distal SCC often presents at earlier stages, so combination therapy may not be necessary to treat the disease, and surgery or radiation alone may be sufficient (3,10,22).
Female PUC
PUC in women is often treated with radical urethrectomy including resection of all surrounding tissue from the bulbocavernosus muscle to the bladder neck with urethral-sparing surgery used if possible to maintain the integrity and function of the urinary tract (3). Partial urethrectomy may be considered over total urethrectomy to maintain urethral integrity; however, one study found a 22% recurrence rate after partial urethrectomy with a 5-year post recurrence survival rate of 71% (24). A radical urethrectomy has shown better prognosis than partial urethrectomy in this patient population (24).
Dalbagni and colleagues (25) showed that combination therapy was more effective than surgery alone at reducing recurrence rates in women with PUC. This coincides with a previous study of both men and women by Son and colleagues (26) that showed combination therapy (with a minimum pelvic radiation dose of 30.6 Gy; excluding debulking surgical procedures) had improved overall survival compared to surgery alone for treating PUC. For early-stage disease, one study noted that combination therapy effectively managed PUC in around one-half of women, but this improvement was nonsignificant (27). This nonsignificant improvement may be disputed from a small single-center retrospective study that showed combination therapy is associated with a 60% disease-free survival rate for patients with later-staged T3+ PUC, but early-stage disease may not require combination therapy for effective treatment (28).
A summary of the studies included in this section are shown in Table 2.
Table 2
| Study | Number of patients | Treatment modality | Outcomes | Length of median follow-up |
|---|---|---|---|---|
| Boorjian, Kim, et al. (2011) (11) | 1,506 | Radical cystectomy | 5.6% UR | 13.5 years |
| Dimarco, Dimarco, et al. (2004) (24) | 26 women | Partial urethrectomy | 50.9% UR; 60% CSS; 42% OS | 12.8 years |
| 27 women | Radical extirpation | |||
| Werntz, Riedinger, et al. (2018) (23) | 725 men | Inguinal lymph node dissection (N1 or N2) | Improved OS (P=0.002) | – |
| Rabbani (2011) (22) | 227 men | Radical excision | Improved CSS (P<0.001) | 2.5 years |
| 78 men | Surgery and radiation | Improved CSS (P=0.017) | ||
| Son, Liauw, et al. (2018) (26) | 1,785 | Surgery | 58% 3-yr OS | 32 months |
| 119 | Radiation | 44% 3-yr OS | 26 months | |
| 302 | Surgery and radiation | 57% 3-yr OS | 30 months | |
| Dalbagni, Donat, et al. (2001) (25) | 6 women | Anterior pelvic exenteration and radiation | 50% metastasis; 33% UR | 21 months |
| Peyton, Azizi, et al. (2018) (27) | 13 women | Multimodal therapy | Longer OS (36 vs. 16 months; P>0.05) | – |
| Gheiler, Tefilli, et al. (1998) (28) | 21 | Neoadjuvant chemotherapy, radiation, with or without surgery | 60% 5-yr CSS | – |
| Smith, Hadway, et al. (2007) (18) | 18 men | Penile preserving surgery | No local recurrence | 26 months |
PUC, primary urethral cancer.
Salvage therapy
Salvage surgery is a treatment option for PUC recurrence. Salvage surgery may involve more radical procedures than first-line options in order to treat the recurrence. Urologists must strongly consider the importance of psychosocial well-being of a patient before performing radical genital surgical procedures (e.g., urethrectomy or penectomy), and reconstructive work should still be considered (16). A recent retrospective study shows similar 3-year overall survival for those receiving salvage surgery, salvage radiotherapy, or a combination of salvage surgery and radiotherapy compared to those who received first-line treatments without recurrence (29).
Chemotherapy, combined chemoradiation, and targeted therapy
Gakis and colleagues studied the effects of chemotherapy on survival in patients with advanced PUC (30). They found that neoadjuvant chemotherapy and neoadjuvant chemoradiotherapy improved overall survival in patients with T3 and/or N+ PUC compared to those patients who were treated with surgery with or without adjuvant chemotherapy. Kent and colleagues (31) supplemented these findings in their own retrospective study of 26 patients with SCC histology and T3+ PUC. In this study, 79% of patients showed complete response to combined chemoradiation; out of these patients who showed complete response, however, 42% had recurrence in a median of 12.5 months. The 5-year disease-free survival rate for these treated individuals was 43.2% (with overall 5-year survival at 52%). Appropriate planning should be considered when developing a treatment regimen as neoadjuvant therapy is significantly associated with 3-year relapse free survival (30).
Targeted therapy has the potential to improve outcomes in urethral cancer. A study by Foundation Medicine (32) identified several clinically relevant genomic alterations in patients with advanced urothelial cancer of the bladder with CDKN2A altered in 34%, FGFR3 altered in 21%, PIK3Ca altered in 20% and ERBB2 altered in 17% of cases. Another study by Foundation Medicine (33) identified clinically relevant genomic alterations in SCC of the penis with CDKN2A altered in 40%, NOTCH1 altered in 25%, PIK3CA altered in 25%, EGFR altered in 20%, CCND1 altered in 20%, BRCA2 altered in 10%, RICTOR altered in 10%, and FBXW7 altered in 10% of cases. Although these studies only involve the urethra through secondary tumor involvement, perhaps there is similar enough pathology between penile and PUC SCC (and/or bladder and PUC UC) where therapies targeting these mutations may have positive outcomes on patients with PUC. Future genomic studies should target PUC to determine if these clinically relevant genomic alterations can be identified in PUC to move towards targeted therapy in treating this rare and often difficult-to-treat malignancy.
Prognosis
A study involving the Surveillance, Epidemiology, and End Results (SEER)—18 registries database consisting of 250 men and 169 women diagnosed with PUC determined that the most common histology for men was UC (53.6%), followed by SCC (34.8%) and AC (11.6%); however, these proportions differed for women with the most common histology being AC (46.7%), followed by SCC (25.4%) and UC (24.9%) (20). In general, women tend to have more advanced tumors of AC type, and men tend to present with UC (12,20,27,34-36). A study of 1,268 men and 869 women with PUC determined a median survival rate of 49 months with a 5-year survival rate of 46% and 10-year survival rate of 31% (34). A small multicenter study noted that proximal SCC had poor prognosis with only survival rate after being treated with pan-urethrectomy and/or prostatectomy; however, this study has limitations due to its retrospective design and small sample size of 10 patients (37).
A large SEER database study by Abudurexiti and colleagues (38) found that men are more likely to have common PUC (1761 men versus 980 women; P<0.001); however, women were more likely to have rare pathological variants (117 men versus 140 women; P<0.001). This study also found a mean overall survival time of 59 months for the common pathological variants and 36 months for the rare pathological variants of PUC. This study noted that there are statistically significant differences between age, T stages and M stages on overall survival, but there were no significant differences between race, gender, and N stages on overall survival.
Recurrence free survival with PUC is significantly associated with clinical nodal stage, tumor location, and age; while overall survival is independently predicted by clinical nodal stage alone (21,35). Thyavihally and colleagues (21) found that survival was much higher for PUC of the distal urethra versus proximal urethral at 72% and 36%, respectively (P=0.02). Detection of asymptomatic PUC is associated with lower stage disease and improved survival when compared to symptomatic PUC (11). Therefore, it seems that early detection is a key component to treating early stage PUC and improving overall survival.
Previous studies debated whether multimodal therapy has any significant benefit to improved overall survival (10,27,37). Eng and colleagues (10) determined that multimodal therapy has better prognosis on more advanced tumor stages, and surgery alone was often a sufficient therapy for early stage tumors; however, one limitation to this study was that the most common histological subtype in their patient population was SCC in both men and women (i.e., this finding may not apply to AC or UC histology). Other studies evaluated whether multimodal therapy has proven benefit to treating PUC, but generalized conclusions seem difficult to make due to difference in outcomes depending on staging, grading, histology, and anatomical difference between sexes. Current studies often have limitations by being retrospective with small sample sizes due to the rarity of the disease (11,20,27,34,35,37).
Conclusions
Surgery to treat PUC is moving from radical procedures such as radical penectomy and radical urethrectomy towards more conservative penile-sparing and urethra-sparing procedures (3,18). Reconstructive procedures must be considered when deciding on radical surgeries (such as a radical penectomy) to preserve penile length and/or urethral structure and function (16). Current literature supports combination therapy when treating late stage PUC in both men and women, but surgery alone is often sufficient for early stage disease (25-28). Salvage surgery and radiation is shown to have similar outcomes as first line therapy in both men and women (16,29). Neoadjuvant chemotherapy or chemoradiotherapy has been shown to improve overall survival in T3+ or N+ patients (30). Targeted therapy has potential, but more studies need to be done in order to identify the altered genes responsible for PUC (32,33). Diagnosis of PUC remains a major hurdle due to the elusiveness of proximal tumors in men to physical exam, the overall lack of symptoms until late-stage disease, and the misdiagnosis of early stage distal tumors (8,11,13,19). Treating PUC is a multi-disciplinary effort involving urologist, gynecologists, radiologists, radiation oncologists, oncologists, and orthopedics in the case of bone metastasis (3). The current literature has limitations due to small sample size and retrospective study designs, so future studies on PUC should aim to be prospective with standardized treatment plans to make a stronger assessment on the effectiveness of different therapies on treating PUC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal for the series “Rare Genitourinary Malignancies”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj.2020.01.06/coif). The series “Rare Genitourinary Malignancies” was commissioned by the editorial office without any funding or sponsorship. PES served as the unpaid Guest Editor of the series and serves as unpaid Associate Editor-in-Chief of AME Medical Journal from September 2017 to February 2020. PES serves as the vice-chair and panel member of the NCCN Bladder and Penile Cancer Committee. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zinman LN, Vanni AJ. Management of proximal primary urethral cancer: should multidisciplinary therapy be the gold standard? Urol Clin North Am 2016;43:505-13. [Crossref] [PubMed]
- Swartz MA, Porter MP, Lin DW, et al. Incidence of primary urethral carcinoma in the United States. Urology 2006;68:1164-8. [Crossref] [PubMed]
- Gakis G, Witjes JA, Comperat E, et al. EAU guidelines on primary urethral carcinoma. Eur Urol 2013;64:823-30. [Crossref] [PubMed]
- Dalbagni G, Zhang ZF, Lacombe L, et al. Female urethral carcinoma: an analysis of treatment outcome and a plea for a standardized management strategy. Br J Urol 1998;82:835-41. [Crossref] [PubMed]
- Traboulsi SL, Witjes JA, Kassouf W. Contemporary management of primary distal urethral cancer. Urol Clin North Am 2016;43:493-503. [Crossref] [PubMed]
- Amin MB, Edge SB. AJCC cancer staging manual. Springer, 2017.
- Edge SB. AJCC cancer staging manual. Springer 2010;7:97-100.
- Stewart SB, Leder RA, Inman BA. Imaging tumors of the penis and urethra. Urol Clin North Am 2010;37:353-67. [Crossref] [PubMed]
- Dalbagni G, Zhang ZF, Lacombe L, et al. Male urethral carcinoma: analysis of treatment outcome. Urology 1999;53:1126-32. [Crossref] [PubMed]
- Eng TY, Chen TW, Patel AJ, et al. Treatment and Outcomes of Primary Urethra Cancer. Am J Clin Oncol 2018;41:905-8. [Crossref] [PubMed]
- Boorjian SA, Kim SP, Weight CJ, et al. Risk factors and outcomes of urethral recurrence following radical cystectomy. Eur Urol 2011;60:1266-72. [Crossref] [PubMed]
- Touijer AK, Dalbagni G. Role of voided urine cytology in diagnosing primary urethral carcinoma. Urology 2004;63:33-5. [Crossref] [PubMed]
- Ryu J, Kim B. MR imaging of the male and female urethra. Radiographics 2001;21:1169-85. [Crossref] [PubMed]
- Hricak H, Secaf E, Buckley DW, et al. Female urethra: MR imaging. Radiology 1991;178:527-35. [Crossref] [PubMed]
- Gourtsoyianni S, Hudolin T, Sala E, et al. MRI at the completion of chemoradiotherapy can accurately evaluate the extent of disease in women with advanced urethral carcinoma undergoing anterior pelvic exenteration. Clin Radiol 2011;66:1072-8. [Crossref] [PubMed]
- Bickell M, Beilan J, Wallen J, et al. Advances in surgical reconstructive techniques in the management of penile, urethral, and scrotal cancer. Urol Clin North Am 2016;43:545-59. [Crossref] [PubMed]
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int 2005;96:1040-3. [Crossref] [PubMed]
- Smith Y, Hadway P, Ahmed S, et al. Penile‐preserving surgery for male distal urethral carcinoma. BJU Int 2007;100:82-7. [Crossref] [PubMed]
- Karnes RJ, Breau RH, Lightner DJ. Surgery for urethral cancer. Urol Clin North Am 2010;37:445-57. [Crossref] [PubMed]
- Aleksic I, Rais-Bahrami S, Daugherty M, et al. Primary urethral carcinoma: A Surveillance, Epidemiology, and End Results data analysis identifying predictors of cancer-specific survival. Urol Ann 2018;10:170. [Crossref] [PubMed]
- Thyavihally YB, Tongaonkar HB, Srivastava SK, et al. Clinical outcome of 36 male patients with primary urethral carcinoma: a single center experience. Int J Urol 2006;13:716-20. [Crossref] [PubMed]
- Rabbani F. Prognostic factors in male urethral cancer. Cancer 2011;117:2426-34. [Crossref] [PubMed]
- Werntz RP, Riedinger CB, Fantus RJ, et al. The role of inguinal lymph node dissection in men with urethral squamous cell carcinoma. Urol Oncol 2018;36:526.e1-526.e6. [Crossref] [PubMed]
- Dimarco DS, DiMarco CS, Zincke H, et al. Surgical treatment for local control of female urethral carcinoma. Urol Oncol 2004;22:404-9. [Crossref] [PubMed]
- Dalbagni G, Donat SM, Eschwege P, et al. Results of high dose rate brachytherapy, anterior pelvic exenteration and external beam radiotherapy for carcinoma of the female urethra. J Urol 2001;166:1759-61. [Crossref] [PubMed]
- Son CH, Liauw SL, Hasan Y, et al. Optimizing the role of surgery and radiation therapy in urethral cancer based on histology and disease extent. Int J Radiat Oncol Biol Phys 2018;102:304-13. [Crossref] [PubMed]
- Peyton CC, Azizi M, Chipollini J, et al. Survival Outcomes Associated With Female Primary Urethral Carcinoma: Review of a Single Institutional Experience. Clin Genitourin Cancer 2018;16:e1003-e1013. [Crossref] [PubMed]
- Gheiler EL, Tefilli MV, Tiguert R, et al. Management of primary urethral cancer. Urology 1998;52:487-93. [Crossref] [PubMed]
- Gakis G, Schubert T, Morgan TM, et al. The prognostic effect of salvage surgery and radiotherapy in patients with recurrent primary urethral carcinoma. Urol Oncol 2018;36:10.e7-10.e14. [Crossref] [PubMed]
- Gakis G, Morgan TM, Daneshmand S, et al. Impact of perioperative chemotherapy on survival in patients with advanced primary urethral cancer: results of the international collaboration on primary urethral carcinoma. Ann Oncol 2015;26:1754-9. [Crossref] [PubMed]
- Kent M, Zinman L, Girshovich L, et al. Combined chemoradiation as primary treatment for invasive male urethral cancer. J Urol 2015;193:532-7. [Crossref] [PubMed]
- Ross JS, Wang K, Khaira D, et al. Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations. Cancer 2016;122:702-11. [Crossref] [PubMed]
- Ali SM, Pal SK, Wang K, et al. Comprehensive genomic profiling of advanced penile carcinoma suggests a high frequency of clinically relevant genomic alterations. Oncologist 2016;21:33-9. [Crossref] [PubMed]
- Sui W. Outcomes and prognostic factors of primary urethral cancer. Urology 2017;100:180-6. [Crossref] [PubMed]
- Gakis G, Morgan TM, Efstathiou JA, et al. Prognostic factors and outcomes in primary urethral cancer: results from the international collaboration on primary urethral carcinoma. World J Urol 2016;34:97-103. [Crossref] [PubMed]
- Derksen JW, Visser O, de la Rivière GB, et al. Primary urethral carcinoma in females: an epidemiologic study on demographical factors, histological types, tumour stage and survival. World J Urol 2013;31:147-53. [Crossref] [PubMed]
- Castiglione F, Alnajjar HM, Christodoulidou M, et al. Primary Squamous Cell Carcinoma of the Male Proximal Urethra: Outcomes from a Single Centre. Eur Urol Focus 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Abudurexiti M, Wang J, Shao N, et al. Prognosis of rare pathological primary urethral carcinoma. Cancer Manag Res 2018;10:6815. [Crossref] [PubMed]
Cite this article as: Carlock HR, Spiess PE. Review on urethral cancer: what do you need to know. AME Med J 2020;5:7.

