
Modelling the deposition of fine particulate matter (PM2.5) in the human respiratory tract
Introduction
In general, particulate matter (PM) or suspended particulate matter (SPM) includes microscopic particles of liquid or solid matter, which are suspended in the atmosphere. These particles can originate either from natural or from anthropogenic sources (1-4). From a physical point of view, PM can be further subdivided into inhalable coarse particles, including particulate objects with a diameter between 2.5 and 10 µm (PM10), fine particles exhibiting a diameter of 2.5 µm or less (PM2.5), ultrafine particles and soot (5-10). In medical respects, all kinds of airborne particles are classified by the IARC and WHO as Group 1 carcinogens (11), whereby most harmful particulates have the ability to penetrate into deep lung regions, where they may reach the blood capillaries unfiltered. This phenomenon, however, may cause heart attacks, respiratory diseases, and premature death (12,13). In 2016, worldwide exposure to PM2.5 contributed to more than 4 million deaths from heart disease and stroke, lung cancer, chronic lung disease, and respiratory infections (14). In total, ambient particulate matter occupies the sixth position among the leading risk factors for premature death globally (15).
Basically, the size of inhaled PM represents the main determinant of where in the respiratory tract highest deposition of particles will take place. Whilst larger particles are already filtered in the nose and the throat, particles smaller than 10 µm can settle in the bronchi and respiratory regions of the lungs (12,13,16-18). Fine particulate matter adopting sizes less than 2.5 µm tends to penetrate into the gas exchange region, whereby smallest components of this inhaled aerosol (PM0.1) may pass the epithelial and subepithelial barriers of the lungs and may affect other organs (19-22). Particles measuring less than 100 nm among other include the so-called Diesel Particulate Matter (DPM). This combustion-derived material, however, is characterized by a large surface area, which allows the adsorption of great amounts of carcinogens (19,23). Medical studies among other could demonstrate that increased uptake of PM2.5 causes high plaque deposits in arteries, resulting in vascular inflammation and atherosclerosis (24). Long-term exposure to fine particulate matter leads to a 13% enhanced risk of heart attacks (25). In 2005, the World Health Organization (WHO) estimated that PM2.5 has to be made responsible for 3% of mortality from cardiopulmonary disease, 5% of mortality from cancer of the trachea, bronchi, and central lungs, and 1% of mortality from acute respiratory infections in children under 5 years, worldwide (13,14,26).
From the detailed explanations made above it can be doubtlessly concluded that PM2.5 may be associated with a multitude of diseases, for what reason precise knowledge of its transport and deposition behaviour in the human respiratory tract is indispensable. Here, theoretical models offer valuable support to continuously increase this level of information. In the present contribution, deposition of PM2.5 taken up into the lungs of different probands (5 years, 10 years, 15 years, and adults) is subject to a detailed theoretical description. Thereby, particles adopting diameters of 100 nm, 0.5 µm, 1.0 µm, 1.5 µm, and 2.0 µm are included into the study.
The hypothetical investigation may be regarded as innovative and necessary insofar as:
- It considers the intrapulmonary transport and deposition behaviour of the particles in both children and adults, allowing the execution of direct comparisons.
- It makes use of most current findings with regard to particle aerodynamics in the respiratory system.
- It is enabled to simulate regional and local deposition scenarios that can be used for the solution of all kinds of medical questions.
Methods
Simulation of particle deposition in the human respiratory tract
The theoretical approach to PM transport and deposition in the human airways and alveoli was carried out by assuming (I) a stochastic structure of the lung with related intrasubject variability of specific geometric parameters (airway length, diameter, branching angle) in each airway generation, (II) particle transport according to a random walk algorithm, where inhaled objects pass different bronchial paths on their way through the lung, and (III) deposition of particles according to well-defined mechanisms (Brownian motion, inertial impaction, interception, gravitational settling). Construction of the stochastic lung architecture was realized by targeted application of probability density functions of the geometric parameters noted above, from which specific values were selected by means of the random number concept (27-30). Hence, the tracheobronchial tree was assembled airway generation by airway generation. Random particle paths were generated by calculation of local probabilities for the entrance of a particulate object into the left and right daughter tube at each airway bifurcation. These probabilities among other depended upon the cross areas of the daughter tubes, the bifurcation angle, and the velocity of the inhaled air stream. Statistical processing of the transport events took place with the help of the Monte Carlo method, where a high number of particles (e.g., 10,000) was inserted into the tracheobronchial tree (27-30). Computation of bronchial and alveolar particle deposition was realized by application of well-validated analytical and empirical formulae for the single deposition mechanisms (27-37). In order to increase the efficiency of the statistical calculations, the mathematical technique of statistical weights was used. This allows the simulation of multiple deposition events for a single particle, whereby after each collision between particle and airway/alveolar wall the statistical weight is reduced by a certain amount (27-30).
Physiological parameters used for modelling
Inhalation of PM2.5 was simulated under the assumption of sitting breathing conditions (38) and uptake of the ambient air through the nose. In total, five size categories (100 nm, 0.5 µm, 1.0 µm, 1.5 µm, and 2.0 µm) were defined, whereby each category contained variably shaped particles with unit-density (1.0 g·cm−3). For the adult respiratory tract a mean functional residual capacity of 3,300 cm3 was assumed, whereas tidal volume was set to 750 cm3 and length of a breath cycle was constituted with 4.2 s (breath-hold: 1.0 s) (38). Respective physiological data of 5-year old, 10-year old and 15-year old children and adolescents were derived from specific scaling functions introduced in previous contributions (30,38-41). Concretely speaking, functional residual capacity adopts values of 757 cm3 (5 years), 1,230 cm3 (10 years), and 2,650 cm3 (15 years), whereas the tidal volume mounts to 244 cm3 (5 years), 456 cm3 (10 years), and 625 cm3 (15 years). Finally, the age-adapted length of the breath cycle can be estimated at 2.0 s (5 years), 2.5 s (10 years), and 3.2 s (15 years), with no breath-hold occurring in the single age groups.
Besides total deposition of PM2.5 denoting the overall accumulation of particles in all parts of the respiratory tract also regional (i.e., tubular and alveolar) deposition as well as generation-by-generation deposition was modelled and subjected to a graphical evaluation.
Results
Total and regional deposition of fine particulate matter
As depicted in Figures 1,2, total and regional deposition of PM2.5 depends on both the size of single aerosol particles and the age of the probands inhaling them. Total deposition of particles with a diameter of 0.1 µm (100 nm) ranges from 18.9% (5 years) to 29.6% (adults) and thus exhibits an increase with proceeding age. This phenomenon, however, can be also observed for larger particles, whereby deposition of objects with a diameter of 0.5 µm ranges from 8.51% (5 years) to 15% (adults). Particles with a diameter of 1.0 µm deposit by 11.9% in the respiratory tract of 5-year-old children, but by 29.9% in the respective system of adults. For particles with a size of 1.5 µm deposition varies between 19.9% and 50.0%, whereas particles measuring 2.0 µm in diameter accumulate in the respiratory structures by 29.8% to 68.4% (Figure 1A).
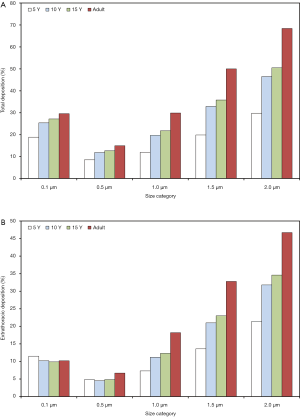
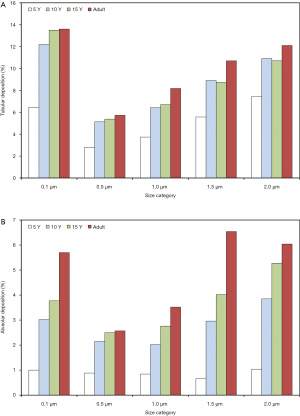
Extrathoracic deposition is characterized by a size dependence and a correlation with subject’s age, which are very similar to those of total deposition. Concretely speaking, 0.1 µm particles deposit in the extrathoracic airways by 11.5% (5 years) to 10.2% (adults), whereas 0.5 µm particles are marked by deposition values ranging from 4.82% to 6.63%. Extrathoracic deposition of particles with a diameter of 1.0 µm amounts to 7.31% to 18.2%, deposition of 1.5 µm particles to 13.6% to 32.8% and deposition of 2.0 µm particles to 21.4% to 46.7% (Figure 1B). Concerning tubular (i.e., bronchial, bronchiolar, and ductal) deposition 0.1 µm particles deposit by 6.46% (5 years) to 13.6% (adults), 0.5 µm particles by 2.8% to 5.73% and 1.0 µm particles by 3.74% to 8.18%. In the case of particles measuring 1.5 µm in size deposition varies between 5.57% and 10.7%, whilst in the case of 2.0 µm particles deposition ranges from 7.44% to 12.1% (Figure 2A). Alveolar deposition of particles with a diameter of 0.1 µm commonly increases from 1.0% (5 years) to 5.7% (adults). Particles measuring 0.5 µm in size deposit by 0.88% to 2.57% in the alveolar structures, whereas 1.0 µm particles exhibit respective deposition values between 0.84% and 3.52%. Particles with a diameter of 1.5 µm accumulate in the alveoli by 0.66% to 6.53%, and particles measuring 2.0 µm in size show deposition values of 1.04% to 6.04% (Figure 2B).
Deposition of fine particulate matter in single airway generations
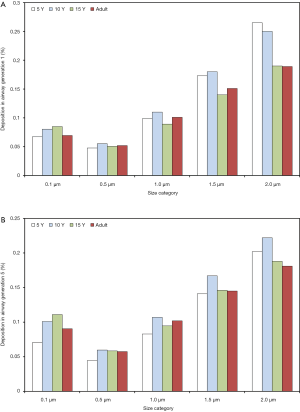
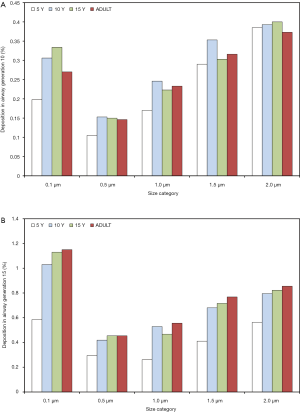
With regard to local (i.e., airway generation-specific) deposition of fine particulate matter, age-related trends are not pronounced in such an unequivocal manner as reported for total and regional deposition. As exhibited in Figures 3,4, local deposition is generally subject to a remarkable decrease from 0.1 µm particles to 0.5 µm particles, but with growing particle size it undergoes a significant enhancement, again. Whilst smallest particles show a continuous increase in deposition with proceeding airway generation, largest particles are mainly accumulated in the upper and central airways, resulting in lower deposition values in the peripheral structures of the respiratory tract. Whilst in the upper and central airway generations young probands (5 and 10 years) commonly produce higher particle depositions, in the peripheral airway generations this behavior is completely turned around with higher deposition rates in adults. In general, particles measuring 0.1 µm in size deposit by 0.067% (generation 1, 5 years) to 1.15% (generation 15, adult), whereas 0.5 µm particles show deposition values between 0.047% and 0.455%. Particles adopting a diameter of 1.0 µm are characterized by a local accumulation ranging from 0.098% to 0.556% and particles with a diameter of 1.5 µm by an accumulation of 0.173% to 0.768%. Finally, particles measuring 2.0 µm in size show a local deposition varying between 0.265% and 0.855% (Figure 3A,B, Figure 4A,B).
Discussion
According to the modelling results fine particulate matter exhibits a specific deposition behavior in the human respiratory tract. Under sitting breathing conditions, total and regional accumulation of PM2.5 depends on (I) the size of single particulate components and (II) the age of the probands inhaling such particles from the ambient air. As computed by the theoretical model, the relationship between deposition fraction on the one hand and particle size on the other can be best described by a U-shaped function with high deposition probabilities occurring for smaller and larger particles and lower probabilities being recognized for intermediately sized particulate objects (27-38). This size dependence of particle deposition, which is also observed for different components of PM2.5, can be considered as result of the circumstance that small particles (<100 nm) mainly undergo a diffusion-controlled settling in the airways and alveoli, whereas large particles (>1.0 µm) are preferentially subject to mass-related deposition phenomena such as inertial impaction and sedimentation. Particulate objects ranging in size between 100 nm and 1 µm, however, offer a minimal working surface for any deposition mechanism, because they are too large for effective Brownian motion, but too small for mass-related phenomena. Hence, deposition of such particles adopts significantly lower values (27-38).
It could be demonstrated that total and regional deposition of variably sized particles belonging to PM2.5 undergoes a continuous increase with proceeding age of the probands. This means that particles are more effectively deposited in adults than in adolescents and children. The main reason for this highly age-specific deposition behavior is founded on the circumstance that (I) children’s lungs are significantly reduced in size with respect to adults’ lungs and (II) breathing habits of children remarkably differ from those of adults. In general, inhalation of children is characterized by low tidal volumes on the one hand and remarkably shortened duration on the other, which in combination with the reduced lung morphometry results in very shallow breath cycles allowing a minor intrapulmonary penetration of the inspired particles and limiting their residence times in the different lung structures. In adult lungs, the contrary effects can be measured, so that inhaled particles are marked by higher penetration depths and elongated intrapulmonary residence times (35-38). It has to be strictly noted in this context that the considerations noted above are related to one single breath cycle. If a specific time span of particle exposure and a breathing frequency of children being about twice as high as that of adults is assumed, particle deposition in children’s lungs clearly exceeds that in adults’ lungs, so that young probands represent the more endangered age group with regard to the hazardous uptake of PM2.5.
Computation of airway generation-specific particle deposition shows that younger probands possess the ability to filter all kinds of particulate objects in the upper and central bronchial tubes with increased efficiency. Contrary, in adult lungs such particles may penetrate in higher amounts to the more peripheral lung regions, where they finally undergo a respective settling event. The modeling results, however, are characterized by several uncertainties, which mainly result from fluctuations with regard to breathing habits and inhaled air volumes. Basically, the results obtained from the theoretical model predict higher particle masses in the lung periphery of adults than in the periphery of children. This would have severe consequences concerning an age-related risk assessment of PM2.5 and the development of appropriate countermeasures.
Finally, it has to be additionally mentioned that particles of arbitrary size deposited in the bronchial/bronchiolar airways and alveolar structures are subjected to an effective innate defense system including faster and slower processes of bronchial and alveolar clearance (30,39-45). These mechanisms are also characterized by a certain age dependence (44,45), so that all physiological processes associated with the inspiratory uptake of particles exhibit a certain difference between children and adults.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj.2020.03.04/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seinfeld J, Spyros P. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. 2nd Ed. Hoboken: John Wiley & Sons; 1998.
- United States Environmental Protection Agency (USEPA). Particulate Matter (PM) Basics. Washington: US EPA; 2016.
- IARC. Diesel and gasoline engine exhausts. In Diesel and Gasoline Engine Exhausts and Some Nitroarenes. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, vol. 46. Lyon: International Agency for Research on Cancer, 1989.
- NTP. Report on Carcinogens Background Document for Diesel Exhaust Particulates. Research Triangle Park: National Toxicology Program, 2000.
- Sturm R. Deposition of diesel exhaust particles in the human lungs: theoretical simulations and experimental data. J Public Health Emerg 2017;1:70. [Crossref]
- Sturm R. Theoretical deposition of random walk-generated nanoaggregates in the lungs of healthy males and females. J Publ Health Emerg 2018;2:4. [Crossref]
- Sturm R. Theoretical deposition of diesel exhaust particles in the respiratory tract of children. J Public Health Emerg 2019;3:12. [Crossref]
- Sturm R. Modellrechnungen zur Deposition nicht-sphärischer Teilchen in den oberen Luftwegen der menschlichen Lunge. Z Med Phys 2009;19:38-46. [Crossref] [PubMed]
- Sturm R. Theoretical models for dynamic shape factors and lung deposition of small particle aggregates originating from combustion processes. Z Med Phys 2010;20:226-34. [Crossref] [PubMed]
- Sturm R. Inhaled nanoparticles. Phys Today 2016;69:70-1. [Crossref]
- Brown JS, Gordon T, Price O, et al. Thoracic and respirable particle definitions for human health risk assessment. Part Fibre Toxicol 2013;10:12. [Crossref] [PubMed]
- Donaldson K, Aitken R, Tran L, et al. Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol Sci 2006;92:5-22. [Crossref] [PubMed]
- Poland CA, Duffin R, Kinloch I, et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 2008;3:423-8. [Crossref] [PubMed]
- McClellan RO. Toxicological effects of emissions from diesel engines. Dev Toxicol Environ Sci 1986;13:3-8. [PubMed]
- Raaschou-Nielsen O, Andersen ZJ, Beelen R, et al. Air pollution and cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). The Lancet Oncology 2013;14:813-22. [Crossref] [PubMed]
- Kasper G. Dynamics and measurement of smokes. I Size characterization of nonspherical particles. Aerosol Sci Technol 1982;1:187-99. [Crossref]
- Sturm R. Inhalation of nanoplatelets - Theoretical deposition simulations. Z med Phys 2017;27:274-84. [Crossref] [PubMed]
- Högberg SM. Modeling nanofiber transport and deposition in human airways. Lulea, Sweden: Tech Univ Lulea, 2010.
- Sturm R. A computer model for the simulation of fiber-cell interaction in the alveolar region of the respiratory tract. Comput Biol Med 2011;41:565-73. [Crossref] [PubMed]
- Sturm R, Hofmann W. A theoretical approach to the deposition and clearance of fibers with variable size in the human respiratory tract. J Hazard Mater 2009;170:210-8. [Crossref] [PubMed]
- Sturm R. Nanotubes in the human respiratory tract - Deposition modelling. Z Med Phys 2015;25:135-45. [Crossref] [PubMed]
- Sturm R. Spatial visualization of theoretical nanoparticle deposition in the human respiratory tract. Ann Transl Med 2015;3:326. [PubMed]
- Sturm R. A stochastic model of carbon nanotube deposition in the airways and alveoli of the human respiratory tract. Inhal Toxicol 2016;28:49-60. [Crossref] [PubMed]
- Sturm R. Theoretical approach to the hit probability of lung-cancer-sensitive epithelial cells by mineral fibers with various aspect ratios. Thoracic Cancer 2010;1:116-25. [Crossref] [PubMed]
- Sturm R, Hofmann W. A computer program for the simulation of fiber deposition in the human respiratory tract. Comput Biol Med 2006;36:1252-67. [Crossref] [PubMed]
- Sturm R. Deposition and cellular interaction of cancer-inducing particles in the human respiratory tract: Theoretical approaches and experimental data. Thoracic Cancer 2010;1:141-52. [Crossref] [PubMed]
- Koblinger L, Hofmann W. Monte Carlo modeling of aerosol deposition in human lungs. Part I: Simulation of particle transport in a stochastic lung structure. J Aerosol Sci 1990;21:661-74. [Crossref]
- Hofmann W, Sturm R, Winkler-Heil R, et al. Stochastic model of ultrafine particle deposition and clearance in the human respiratory tract. Radiat Prot Dosimetry 2003;105:77-80. [Crossref] [PubMed]
- Sturm R. A computer model for the simulation of nanoparticle deposition in the alveolar structures of the human lungs. Ann Transl Med 2015;3:281. [PubMed]
- Sturm R. Theoretical models of carcinogenic particle deposition and clearance in children’s lungs. J Thorac Dis 2012;4:368-76. [PubMed]
- Sturm R. Modeling the deposition of bioaerosols with variable size and shape in the human respiratory tract - A review. J Adv Res 2012;3:295-304. [Crossref]
- Sturm R. Bioaerosols in the lungs of subjects with different ages - part 1: deposition modeling. Ann Transl Med 2016;4:211. [Crossref] [PubMed]
- Willeke K, Baron PA. Aerosol measurement. New York: John Wiley; 1993.
- Ingham DB. Diffusion of aerosol from a stream flowing through a cylindrical tube. J Aerosol Sci 1975;6:125-32. [Crossref]
- Yeh HC, Schum GM. Models of the human lung airways and their application to inhaled particle deposition. Bull Math Biol 1980;42:461-80. [Crossref] [PubMed]
- Zhang L, Asgharian B, Anjilvel S. Inertial and interceptional deposition of fibers in a bifurcating airway. J Aerosol Med 1996;9:419-30. [Crossref] [PubMed]
- Sturm R. Theoretical deposition of carcinogenic particle aggregates in the upper respiratory tract. Ann Transl Med 2013;1:25. [PubMed]
- International Commission on Radiological Protection (ICRP). Human respiratory tract model for radiological protection, Publication 66. Oxford: Pergamon Press; 1994.
- Sturm R. Theoretical and experimental approaches to the deposition and clearance of ultrafine carcinogens in the human respiratory tract. Thoracic Cancer 2011;2:61-8. [Crossref] [PubMed]
- Sturm R. Theoretical models for the simulation of particle deposition and tracheobronchial clearance in lungs of patients with chronic bronchitis. Ann Transl Med 2013;1:3. [PubMed]
- Sturm R. Carbon nanotubes in the human respiratory tract – clearance modeling. Ann Work Expo Health 2017;61:226-36. [Crossref] [PubMed]
- Sturm R. An advanced stochastic model for mucociliary particle clearance in cystic fibrosis lungs. J Thorac Dis 2012;4:48-57. [PubMed]
- Hofmann W, Sturm R, Asgharian B. Stochastic simulation of particle clearance in human bronchial airways. J Aerosol Sci 2001;2S807-8.
- Sturm R. Bioaerosols in the lungs of subjects with different ages – Part 2: clearance modeling. Ann Transl Med 2017;5:95. [Crossref] [PubMed]
- Sturm R. Age-dependence and intersubject variability of tracheobronchial particle clearance. Pneumon 2011;4:77-84.
Cite this article as: Sturm R. Modelling the deposition of fine particulate matter (PM2.5) in the human respiratory tract. AME Med J 2020;5:14.

