
The effect of perioperative blood transfusion on oncological outcomes in radical cystectomy patients: a narrative review
Introduction
Bladder cancer (BCa) is the 6th most common cancer worldwide (1), the second in the genitourinary system with an estimated 80,470 new cases in 2019, and the 9th leading cause of cancer death (2). The majority of BCa is diagnosed after the occurrence of haematuria, with 75% of patients who presents a non-muscle invasive disease (3). However, these patients have a high risk of recurrence (50% of cases) and 20% of risk of progression at 5 years (4). Radical cystectomy (RC) with bilateral pelvic lymph node dissection (PLND) (3) represents the standard of care of very high-risk non-muscle invasive BCa and of muscle-invasive BCa. Nowadays, open radical cystectomy (ORC) is the most commonly performed surgical technique: however, in the last decade, minimally invasive surgical approaches including laparoscopic (LRC) or robotic radical cystectomy (RARC) (5) have spread worldwide. Although the introduction of these procedures, RC remains a complex surgery, burdened by high rates of perioperative morbidity and mortality: about 60% of the cases suffers from at least one complication within 90 days after surgery (6), and 30- and 90-day postoperative mortality rates are around 3% and 7%, respectively (7). Among the most common complications, there is intraoperative bleeding, which can require or not blood transfusions (BTs). This complication could be attributed to two main factors: first of all, to the technical complexity of the procedure and, secondly, to patients’ population which usually includes elderly patients with significant comorbidities. Moreover, the neoplasm itself can bleed, causing preoperative anaemia which can increase the risk of postoperative complications and the need of transfusions. Perioperative transfusion rate in patients undergoing RC is around 60% (8,9). Several studies suggested that perioperative BTs might have an impact on survival outcomes in RC patients but results reported in literature are controversial. For this reason, we sought to review the current available studies to evaluate the association between allogeneic blood transfusions (ABTs) and survival outcomes in patients treated with RC and PLND with curative intent for BCa.
Evidence acquisition
We searched the Medline/PubMed database using individual or/and different combinations of terms including: “bladder cancer”, “urothelial carcinoma of the bladder”, “radical cystectomy”, “perioperative blood transfusion”, “cancer recurrence”, “survival”, “oncological outcomes” and “mortality”. Only title and abstract in English language were screened for eligibility: if included, the full text was analyzed. Our research included original article and meta-analyses from 2012 to 2019.
The effect of transfusion in surgical patients
Despite the potential life-saving role, BTs could be related to significant complications including transfusion-associated lung injury (TRALI), transmission of infections, and allergic reactions. For these reasons, over the past 40 years, several studies focused their attention on the effect of ABT in patients treated with surgery, identifying both proinflammatory and immunosuppressive effects. The first observations date back in 1973, when Opelz et al. (10) reported improved survival rates in renal-transplanted patients who received ABT compared to those who did not. Other observational studies underlined a role of ABT in decreasing the risk of recurrence in autoimmune disorders (such as Crohn’s disease) (11) and in spontaneous abortions in women with a history of recurrent abortions (12). On the other side, this immunosuppressive role can lead to deleterious effects: in 1981 Gantt et al. (13) suggested a possible association between ABT and increased risk of cancer recurrence and metastases due to the dysregulated recipient’s immune system. Other harmful effects include an increased risk of postoperative bacterial infections (14) and activation of latent CMV and HIV infections (15).
Several studies tried to clarify the mechanisms of transfusion-related immunomodulation (TRIM) (16). The TRIM effect is mediated by: (I) immunologically active white blood cells (WBC) that downregulate the recipient’s immune system by shifting to immunosuppressive Lymphocytes Th2 responses (17); (II) soluble WBC-derived mediators that induce innate immune cell apoptosis and decrease natural killer cell activity (18); (III) platelet (PLT) and PLT-derived factors; (IV) heme and iron derived by aged and damaged red blood cell (RBC) (named as “storage lesions”) (19); finally (V) ubiquitin and (VI) extracellular vesicle (EV) counts which increase with storage duration (20). This mechanism is depicted in Figure 1.
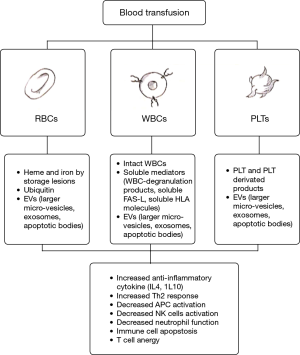
Moreover, the intra-operative release of circulating tumor cells caused by surgical manipulation (21) and the decrease of host’s immune system due to anaesthetics and opioids (22), could have an impact on oncological outcomes in patients treated with perioperative blood transfusions. These association between ABT and worse survival has been investigated in various malignancies, such as colorectal (23), hepatic (24), esophageal (25) and pancreatic cancer (26). In the urological field, contradictory data have been reported among patients with kidney (27,28), prostate (29,30) and BCa and the impact of ABT in these cancers is not yet clarified.
The oncological effect of transfusion in patients who underwent RC
The studies evaluating the effect of perioperative ABT in BCa patients treated with RC are summarized in Table 1. Linder et al. (8) in 2013 analyzed 2,060 patients treated with RC: of them, 1,279 received ABT (62%). At multivariable analyses ABT was found associated with an increased risk of tumor recurrence [hazard ratio (HR): 1.20, confidence interval 95% (CI): 1.01–1.42; P value =0.04], of cancer-specific mortality (HR: 1.31, 95% CI: 1.10–1.57; P=0.003) and of all-causes mortality (HR: 1.27, 95% CI: 1.12–1.45; P=0.0002). Similar results were reported by Buchner et al. (31) who analyzed a cohort of patients treated with RC in a retrospective single-center study. Of the 722 patients included in the analyses, 473 received ABT which was found significantly associated with a decreased cancer-specific survival (HR: 1.11, 95% CI: 1.06–1.16; P<0.001). The authors performed a sub-analysis, dividing BT into two groups: intraoperative blood transfusion (IBT) and postoperative blood transfusion (PBT): both variables remained significantly associated with reduced cancer specific survival with an HR: 1.08, 95% CI: 1.01–1.15; P=0.23 for IBT and an HR: 1.14, 95% CI: 1.07–1.21; P<0.001 for PBT. Similarly, Syan-Bhanvadia et al. (32) found an association between ABT and reduced recurrence-free survival (HR: 2.16, 95% CI: 1.13–41.12; P=0.02) and overall survival (HR 2.25, 95% CI: 1.25–4.88; P=0.01). The authors also suggested a restrictive transfusion protocol which could be safer for patients treated with RC. Similar results were reported in Siemens et al. study (33), in which 2,593 patients who underwent RC between 2000 and 2008 were analyzed. Of them, 62% received ABT which was found associated with worse overall survival (HR: 1.33, 95% CI: 1.20–1.48; P<0.001) and cancer-specific survival at 5 years (HR: 1.39, 95% CI: 1.23–1.56; P<0.001).
Table 1
| Study | Year of publication | Study design | Number of patients | Transfusion group, n (%) | FU (months) | Type of analysis | Outcomes | Results | P value |
|---|---|---|---|---|---|---|---|---|---|
| Linder et al. (8) | 2013 | Retrospective, single-center | 2,060 | 1,279 (62%) | 131 | MVA cox regression analysis | CSM; OM; recurrence | HR: 1.31, CI: 1.10–1.57 | 0.003 |
| HR: 1.27, CI: 1.12–1.45 | 0.0002 | ||||||||
| HR: 1.20, CI: 1.01–1.42 | 0.04 | ||||||||
| Gierth et al. (9) | 2014 | Retrospective, single-center | 350 | Overall 219 (63%): 183 IBT; 99 PBT; 63 IBT + PBT | 70 | MVA cox regression analysis | RFS for IBT; RFS for PBT; OS for IPB; OS for PBT | HR: 1.50, CI: 1.27–1.77 | <0.001 |
| HR: 1.56, CI: 1.30–1.88 | <0.001 | ||||||||
| HR: 1.77, CI: 1.47–2.13 | <0.001 | ||||||||
| HR: 1.76, CI: 1.41–2.21 | <0.001 | ||||||||
| Buchner et al. (31) | 2017 | Retrospective, single-center | 722 | Overall 473 (66%): 263 IBT; 132 PBT; 78 IBT + PBT | 26 | MVA cox regression analysis | CSS for IBT; CSS for PBT | HR:1.08, CI: 1.01–1.15 | 0.23 |
| HR: 1.14, CI: 1.07–1–21 | <0.001 | ||||||||
| Syan-Bhanvadia et al. (32) | 2017 | Prospective, single-center | 173 | 46 (27%) | 37 | MVA cox regression analysis | RFS; OS | HR: 2.16, CI: 1.13–41.12 | 0.02 |
| HR: 2.25, CI: 1.25–4.88 | 0.01 | ||||||||
| Siemens et al. (33) | 2017 | Retrospective, single-center | 2,593 | 1,608 (62%) | – | MVA cox regression analysis | CSS; OS | HR: 1.33, CI: 1.20–1.48 | <0.001 |
| HR: 1.39, CI: 1.23–1.56 | <0.001 | ||||||||
| Morgan et al. (34) | 2013 | Retrospective, single-center | 777 | 323 (42%) | 25.0 | Non-transformed model; Restricted cubic splines model | OM | HR: 1.17, CI: 1.01–1.36 | 0.04 |
| HR: 1.03, CI: 0.77–1.37 | 0.29 | ||||||||
| Soubra et al. (35) | 2015 | Retrospective, multicenter | 5,462 | 1,116 (20%) | 21 | MVA cox regression analysis | CSM; OM | HR: 1.05, CI: 0.91–1.20 | 0.4 |
| HR: 1.10, CI: 1.01–1.21 | 0.02 | ||||||||
| Kluth et al. (36) | 2014 | Retrospective, multicenter | 2,895 | 1,128 (39%) | 36.1 | MVA cox regression analysis | CSM; OM; recurrence | HR: 1.10, CI: 0.96–1.27 | 0.17 |
| HR: 1.10, CI: 0.99–1.22 | 0.07 | ||||||||
| HR: 1.13, CI: 0.99–1.28 | 0.06 | ||||||||
| Lee et al. (37) | 2015 | Retrospective, single-center | 432 | 315 (73%) | 39.5 | MVA cox regression analysis | OS | HR: 1.56, CI: 0.98–2.48 | 0.058 |
| Vetterlein et al. (38) | 2018 | Prospective, single-center | 611 | 315 (52%) | 26 | MVA cox regression analysis and MVA competing-risk analysis | CSM; OS; recurrence | SHR: 1.03, CI: 0.57–1.87 | >0.9 |
| HR: 1.34, CI: 0.90–1.99 | 0.02 | ||||||||
| HR: 0.96, CI: 0.54–1.70 | 0.9 | ||||||||
| Abel et al. (39) | 2014 | Retrospective, multicenter | 360 (UW); 1,770 (Mayo Clinic) |
Overall 241 (67%): 66 IBT; 79 PBT; 98 IBT + PBT. Overall 1,100 (62%): 414 IBT; 285 only PBT; 401 IBT + PBT | 18.7; 132 | MVA cox regression analysis | CSM for IBT; CSM for PBT; OM for IBT; OM for PBT; Recurrence for IBT; Recurrence for PBT. CSM for IBT; CSM for PBT; OM for IBT; OM for PBT; Recurrence for IBT; Recurrence for PBT | HR: 1.49, CI: 1.00–2.25 | 0.056 |
| HR: 0.91, CI: 0.54–1.53 | 0.7 | ||||||||
| HR: 1.40, CI: 1.20–1.62 | <0.0001 | ||||||||
| HR: 1.06, CI: 0.88–1.27 | 0.56 | ||||||||
| HR: 1.45, CI: 0.84–2.5 | 0.18 | ||||||||
| HR: 1.11, CI: 0.69–1.19 | 0.76 | ||||||||
| HR: 1.55, CI: 1.24–1.94 | 0.0001 | ||||||||
| HR: 0.89, 0.67–1.18 | 0.41 | ||||||||
| HR: 1.40, CI: 1.20–1.62 | <0.0001 | ||||||||
| HR: 1.06, CI: 0.88–1.27 | 0.56 | ||||||||
| HR:1.4, CI: 1.16–1.81 | 0.001 | ||||||||
| HR: 0.91, CI: 0.68–1.20 | 0.49 | ||||||||
| Moschini et al. (40) | 2015 | Retrospective, single-center | 1,490 | Overall 580 (39%): 322 IBT 97 PBT 161 IBT + PBT |
125 | MVA cox regression analysis | Recurrence for IBT; Recurrence for PBT; CSM for IBT; CSM for PBT; OM for IBT; OM for PBT | HR: 1.24, CI: 1.03–1.65 | 0.04 |
| HR: 1.50, CI: 0.78–2.89 | 0.5 | ||||||||
| HR: 1.60, CI: 1.20–2.26 | 0.02 | ||||||||
| HR: 1.60, CI: 0.81–3.17 | 0.2 | ||||||||
| HR: 1.45, CI: 1.02–2.08 | 0.03 | ||||||||
| HR: 1.36, CI: 0.72–2.60 | 0.4 | ||||||||
| Moschini et al. (41) | 2016 | Retrospective, single-center | 728 | – | 97 | MVA cox regression analysis | Recurrence for IBT; Recurrence for PBT | HR: 1.43, CI: 1.15–1.97 | 0.03 |
| HR: 1.83, CI: 0.92–3.01 | 0.1 | ||||||||
| Moschini et al. (42) | 2017 | Retrospective, single-center | 1,081 (testing cohort); 433 (validation cohort) | Overall 445 (42%): 274 IBT; 76 PBT; 122 IBT + PBT. Overall 183 (42%): 122 IBT; 28 PBT; 28 IBT + PBT. | 52; 83 | MVA cox regression analysis | Distant recurrence for IBT; Distant recurrence for PBT; Distant recurrence for IBT; Distant recurrence for PBT | HR: 1.15, CI: 0.74–1.78 | 0.5 |
| HR: 1.32, CI: 0.84–2.05 | 0.2 | ||||||||
| HR: 1.22, CI: 0.6–2.46 | 0.6 | ||||||||
| HR: 1.55, CI: 0.84–2–87 | 0.4 | ||||||||
| Sadeghi et al. (43) | 2012 | Retrospective, single-center | 638 | 209 (33%) | 25.5 | MVA cox regression analysis | CSS OS |
HR: 1.2, CI: 0.85–1.69 | 0.3 |
| HR: 1.15, CI: 0.91–1.45 | 0.246 |
FU, follow up; MVA, multivariable; CSM, cancer-specific mortality; HR, hazard ratio; CI, confidence interval; OM, overall mortality; IBT, intraoperative blood transfusion; PBT, postoperative blood transfusion; RFS, recurrence-free survival; OS, overall survival; CSS, cancer-specific survival; SBH, sub-hazard ratio.
However, Morgan et al. (34) reported conflicting results, depending on the statistical method used for the analyses: in a non-transformed model (in which continuous variables were assumed to have linear relationships with the outcomes), the authors found that ABT (n=323, 41.6%) was associated with a significant higher risk of overall mortality (HR: 1.17; P=0.04). On the contrary, in the second model (a restricted cubic splines model for nonlinear relationships) no association was found between them (HR: 1.03; P=0.29). Soubra et al. (35) analyzed the relationship between ABT and mortality in patients who underwent surgical treatment for major urologic cancers, such as bladder, prostate and kidney cancer. In the BCa cohort, the authors reported a significant association between ABT and increased all-causes mortality (HR: 1.109, 95% CI: 1.011–1.21; P=0.028), whereas no significant association between ABT and cancer-specific mortality was reported (HR: 1.052, 95% CI: 0.919–1.204; P=0.4648). Kluth et al. (36), in a multicenter retrospective study, did not find an association between ABT and worse oncological outcomes in the multivariable analysis (disease recurrence p = 0.06, cancer-specific mortality P=0.17, any-cause mortality P=0.07). Similarly, in a retrospective single-center study, Lee et al. (37) compared patients who received ABT (315, 73% of all patients) to those who did not and no significant association was found between ABT and overall survival in the multivariable analysis (HR: 1.56, 95% CI: 0.98–2.48; P=0.058). Similarly, Vetterlein et al. (38) recorded data from 611 patients underwent RC in 2011, of whom 315 (52%) received ABT. The authors found that ABT was not an independent predictor of oncological outcomes, including disease recurrence (HR: 0.96, 95% CI: 0.54–1.70; P=0.9), overall survival (HR: 1.34, 95% CI: 0.90–1.99; P=0.2), cancer-specific mortality (sub-hazard ratio (SHR):1.03, 95% CI: 0.57–1.87; P>0.9) and other-cause mortality (SHR: 2.16, 95% CI: 0.99–4.74; P=0.054).
Finally, there are only two systematic reviews, published by Wang et al. (44) in 2015 and by Cata et al. (45) in 2016. In the first meta-analysis ABT was an independent factor to predict all-causes mortality, cancer-specific mortality and cancer recurrence. Similarly, Cata et al. (45) found a significant association between ABT and cancer-specific survival, overall survival and recurrence-free survival.
Effect of timing of blood transfusion on survival
Few data exist regarding the role of the timing of ABT, considered as IBT or PBT.
Gierth et al. (9) collected data from 350 patients treated with RC. Overall, 219 patients were treated with ABT and 183 (52%) received IBT, whereas 99 (28%) PBT. The authors showed that both IBT and PBT are significant independent predictor of progression-free survival (HR: 1.50, 95% CI: 1.27–1.77; P<0.001 and HR: 1.56, 95% CI: 1.30–1.88; P<0.001 for IBT and PBT, respectively) and overall survival (HR: 1.77, 95% CI: 1.47–2.13; P<0.001 and HR: 1.76, 95% CI: 1.41–2.21; P<0.001 for IBT and PBT, respectively). On the contrary, Buchner et al. (31) reported that PBT was associated with a decrease in cancer-specific survival (HR: 1.14, 95% CI: 1.07–1.21; P<0.001), whereas IBT was not significant (HR: 1.08, 95% CI: 1.01–1.15; P=0.23). Abel et al. analyzed two different cohorts of patients treated with RC: a primary cohort of 360 patients from University of Wisconsin (UW) and a validation cohort of 1,770 patients from Mayo Clinic and patients were divided into a group which received IBT and a group which received PBT. In the primary cohort, the authors found that IBT was an independent risk factor for cancer-specific mortality (HR: 1.77, 95% CI: 1.06–2.94; P=0.03), while PBT was not associated with worse survival outcomes. No significant relationship was found for intra and PBT regarding tumor recurrence and all-causes mortality in the same cohort. Moreover, in the validation cohort from Mayo Clinic, IBT was found associated with a significant higher risk of tumor recurrence (HR: 1.45, 95% CI: 1.16–1.81; P=0.001), cancer-specific mortality (HR: 1.55, 95% CI 1.24–1.94; P=0.0001) and all-causes mortality (HR: 1.40, 95% CI: 1.20–1.62; P<0.0001), while PBT was not associated with worsening prognosis. Similarly, Moschini et al. (40) recorded data from 1,490 patients who underwent RC between 1990 and 2013. Of them, 322 patients received IBT, 97 received PBT and 161 received both IBT and PBT. In the multivariable analysis patients who received IBT and both IBT and PBT were combined in a single group. The authors found that IBT was an independent risk factor for cancer-specific mortality (HR: 1.6, 95% CI: 1.20–2.26; P=0.02), all-causes mortality (HR: 1.45, 95% CI: 1.02–2.08; P=0.03) and tumor recurrence (HR: 1.24, 95% CI: 1.03–1.65; P=0.04). On the contrary, the administration of PBT was not associated with worse oncological outcomes. The same result was found in another study (41), in which IBT was found significantly associated with cancer-specific mortality and overall mortality, whereas no association was found for PBT (P>0.05). Moreover, Moschini et al. (42) in another study, evaluated the risk of distant recurrence after RC in two independent cohorts of patients (testing and validation cohort), considering patients according timing of administration of ABT (IBT vs. PBT). In both cohorts, timing of BT was not significantly related to an increased risk of distant recurrence (all P≥0.2).
Number of units transfused
Only a few studies investigated the relationship between number of units transfused and survival outcomes of patients treated with RC.
Linder et al. (8) found a positive association between number of units transfused and increased risk of cancer-specific mortality (HR: 1.07; P<0.0001) and all-causes mortality (HR 1.05; P<0.0001): each blood’s unit received was associated with a 7% increased risk of cancer-specific mortality. Likewise, Lee et al. (37) recorded that an increased number of units transfused (i.e., >4 units) was a significant independent predictor of overall survival (HR: 1.69, 95% CI: 1.15–2.49; P=0.007). Abel et al. (39) reported that among patients who received an IBT in the primary cohort from University of Wisconsis, each unit transfused conferred a 17% increased risk of cancer-specific mortality (HR: 1.17, 95% CI: 1.03–1.32; P=0.01), whereas no association was found among patients who received PBT in the same cohort (HR: 1.05, 95% CI: 0.72–1.54; P=0.8). Similar results were reported for the validation cohort from Mayo Clinic (HR: 1.07, 95% CI: 1.03–1.11; P=0.0001 for IBT and HR: 0.92, 95% CI: 0.79–1.06; P=0.26 for PBT). Similarly, Gierth et al. (9) found a worse prognosis in terms of progression-free survival and overall survival the more blood units were transfused (P<0.001 for IBT and PBT).
On the contrary, Sadeghi et al. (43) analyzed data from 638 patients: of them 209 (33%) received ABT. On multivariable analysis the number of units transfused was not an independent factor to predict cancer-specific survival (P=0.3) and overall survival (P=0.246). In Moschini et al. (42) study, the number of unit transfused was not found associated with an increased risk of distant recurrence.
Conclusions
RC represents a complex surgery, which often requires BTs. Several studies have investigated the effects of perioperative blood transfusions in patients with BCa treated with RC, especially in terms of oncological outcomes, investigating also the correct timing of perioperative blood transfusions. Unfortunately, the relationship between ABT and survival outcomes is still unclear, with contrasting results reported in literature: further studies are needed to explain this complex relationship in order to address the medical practice to an individualized treatment and to improve prognosis of these fragile patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal for the series “Bladder Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj.2020.02.03/coif). The Series “Bladder Cancer” was commissioned by the editorial office without any funding or sponsorship. MM served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of AME Medical Journal from September 2019 to August 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Antoni S, Ferlay J, Soerjomataram I, et al. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol 2017;71:96-108. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol 2017;71:462-75. [Crossref] [PubMed]
- Cambier S, Sylvester RJ, Collette L, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guérin. Eur Urol 2016;69:60-9. [Crossref] [PubMed]
- Zamboni S, Soria F, Mathieu R, et al. Differences in trends in the use of robot-assisted and open radical cystectomy and changes over time in peri-operative outcomes among selected centres in North America and Europe: an international multicentre collaboration. BJU Int 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009;55:164-74. [Crossref] [PubMed]
- Hanna N, Leow JJ, Sun M, et al. Comparative effectiveness of robot-assisted vs. open radical cystectomy. Urol Oncol 2018;36:88.e1-88.e9. [Crossref] [PubMed]
- Linder BJ, Frank I, Cheville JC, et al. The impact of perioperative blood transfusion on cancer recurrence and survival following radical cystectomy. Eur Urol 2013;63:839-45. [Crossref] [PubMed]
- Gierth M, Aziz A, Fritsche HM, et al. The effect of intra- and postoperative allogenic blood transfusion on patients’ survival undergoing radical cystectomy for urothelial carcinoma of the bladder. World J Urol 2014;32:1447-53. [Crossref] [PubMed]
- Opelz G, Sengar DP, Mickey MR, et al. Effect of blood transfusions on subsequent kidney transplants. Transplant Proc 1973;5:253-9. [PubMed]
- Peters WR, Fry RD, Fleshman JW, et al. Multiple blood transfusions reduce the recurrence rate of Crohn’s disease. Dis Colon Rectum 1989;32:749-53. [Crossref] [PubMed]
- Mowbray JF, Gibbings C, Liddell H, et al. Controlled trial of treatment of recurrent spontaneous abortion by immunisation with paternal cells. Lancet 1985;1:941-3. [Crossref] [PubMed]
- Gantt CL. Red blood cells for cancer patients. Lancet 1981;2:363. [Crossref] [PubMed]
- Rohde JM, Dimcheff DE, Blumberg N, et al. Health care-associated infection after red blood cell transfusion: a systematic review and meta-analysis. JAMA 2014;311:1317-26. [Crossref] [PubMed]
- Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev 2007;21:327-48. [Crossref] [PubMed]
- Vamvakas EC. Meta-analysis of randomized controlled trials investigating the risk of postoperative infection in association with white blood cell-containing allogeneic blood transfusion: the effects of the type of transfused red blood cell product and surgical setting. Transfus Med Rev 2002;16:304-14. [Crossref] [PubMed]
- Remy KE, Hall MW, Cholette J, et al. Mechanisms of red blood cell transfusion-related immunomodulation. Transfusion 2018;58:804-15. [Crossref] [PubMed]
- Ghio M, Contini P, Ubezio G, et al. Blood transfusions with high levels of contaminating soluble HLA-I correlate with levels of soluble CD8 in recipients’ plasma; a new control factor in soluble HLA-I-mediated transfusion-modulated immunomodulation? Blood Transfus 2014;12:s105-108. [PubMed]
- D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion 2015;55:205-19. [Crossref] [PubMed]
- Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 2004;104:2543-8. [Crossref] [PubMed]
- Juratli MA, Sarimollaoglu M, Siegel ER, et al. Real-time monitoring of circulating tumor cell release during tumor manipulation using in vivo photoacoustic and fluorescent flow cytometry. Head Neck 2014;36:1207-15. [Crossref] [PubMed]
- Kavanagh T, Buggy DJ. Can anaesthetic technique effect postoperative outcome? Curr Opin Anaesthesiol 2012;25:185-98. [Crossref] [PubMed]
- Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev 2006;CD005033. [PubMed]
- Wang CC, Iyer SG, Low JK, et al. Perioperative factors affecting long-term outcomes of 473 consecutive patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg Oncol 2009;16:1832-42. [Crossref] [PubMed]
- Motoyama S, Okuyama M, Kitamura M, et al. Use of autologous instead of allogeneic blood transfusion during esophagectomy prolongs disease-free survival among patients with recurrent esophageal cancer. J Surg Oncol 2004;87:26-31. [Crossref] [PubMed]
- Kneuertz PJ, Patel SH, Chu CK, et al. Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol 2011;18:1327-34. [Crossref] [PubMed]
- Moffat LE, Sunderland GT, Lamont D. Blood transfusion and survival following nephrectomy for carcinoma of kidney. Br J Urol 1987;60:316-9. [Crossref] [PubMed]
- Linder BJ, Thompson RH, Leibovich BC, et al. The impact of perioperative blood transfusion on survival after nephrectomy for non-metastatic renal cell carcinoma (RCC). BJU Int 2014;114:368-74. [PubMed]
- Ford BS, Sharma S, Rezaishiraz H, et al. Effect of perioperative blood transfusion on prostate cancer recurrence. Urol Oncol 2008;26:364-7. [Crossref] [PubMed]
- Heal JM, Chuang C, Blumberg N. Perioperative blood transfusions and prostate cancer recurrence and survival. Am J Surg 1988;156:374-80. [Crossref] [PubMed]
- Buchner A, Grimm T, Schneevoigt BS, et al. Dramatic impact of blood transfusion on cancer-specific survival after radical cystectomy irrespective of tumor stage. Scand J Urol 2017;51:130-6. [Crossref] [PubMed]
- Syan-Bhanvadia S, Drangsholt S, Shah S, et al. Restrictive transfusion in radical cystectomy is safe. Urol Oncol 2017;35:528.e15-528.e21. [Crossref] [PubMed]
- Siemens DR, Jaeger MT, Wei X, et al. Peri-operative allogeneic blood transfusion and outcomes after radical cystectomy: a population-based study. World J Urol 2017;35:1435-42. [Crossref] [PubMed]
- Morgan TM, Barocas DA, Chang SS, et al. The relationship between perioperative blood transfusion and overall mortality in patients undergoing radical cystectomy for bladder cancer. Urol Oncol 2013;31:871-7. [Crossref] [PubMed]
- Soubra A, Zabell JR, Adejoro O, et al. Effect of perioperative blood transfusion on mortality for major urologic malignancies. Clin Genitourin Cancer 2015;13:e173-181. [Crossref] [PubMed]
- Kluth LA, Xylinas E, Rieken M, et al. Impact of peri-operative blood transfusion on the outcomes of patients undergoing radical cystectomy for urothelial carcinoma of the bladder. BJU Int 2014;113:393-8. [Crossref] [PubMed]
- Lee JS, Kim HS, Jeong CW, et al. The prognostic impact of perioperative blood transfusion on survival in patients with bladder urothelial carcinoma treated with radical cystectomy. Korean J Urol 2015;56:295-304. [Crossref] [PubMed]
- Vetterlein MW, Gild P, Kluth LA, et al. Peri-operative allogeneic blood transfusion does not adversely affect oncological outcomes after radical cystectomy for urinary bladder cancer: a propensity score-weighted European multicentre study. BJU Int 2018;121:101-10. [Crossref] [PubMed]
- Abel EJ, Linder BJ, Bauman TM, et al. Perioperative blood transfusion and radical cystectomy: does timing of transfusion affect bladder cancer mortality? Eur Urol 2014;66:1139-47. [Crossref] [PubMed]
- Moschini M, Dell’Oglio P, Capogrosso P, et al. Effect of Allogeneic Intraoperative Blood Transfusion on Survival in Patients Treated With Radical Cystectomy for Nonmetastatic Bladder Cancer: Results From a Single High-Volume Institution. Clin Genitourin Cancer 2015;13:562-7. [Crossref] [PubMed]
- Moschini M, Bianchi M, Rossi MS, et al. Timing of blood transfusion and not ABO blood type is associated with survival in patients treated with radical cystectomy for nonmetastatic bladder cancer: Results from a single high-volume institution. Urol Oncol 2016;34:256.e7-256.e13. [Crossref] [PubMed]
- Moschini M, Soria F, Abufaraj M, et al. Impact of Intra- and Postoperative Blood Transfusion on the Incidence, Timing, and Pattern of Disease Recurrence After Radical Cystectomy. Clin Genitourin Cancer 2017;15:e681-8. [Crossref] [PubMed]
- Sadeghi N, Badalato GM, Hruby G, et al. The impact of perioperative blood transfusion on survival following radical cystectomy for urothelial carcinoma. Can J Urol 2012;19:6443-9. [PubMed]
- Wang YL, Jiang B, Yin FF, et al. Perioperative Blood Transfusion Promotes Worse Outcomes of Bladder Cancer after Radical Cystectomy: A Systematic Review and Meta-Analysis. PloS One 2015;10:e0130122. [Crossref] [PubMed]
- Cata JP, Lasala J, Pratt G, et al. Association between Perioperative Blood Transfusions and Clinical Outcomes in Patients Undergoing Bladder Cancer Surgery: A Systematic Review and Meta-Analysis Study. J Blood Transfus 2016;2016:9876394. [Crossref] [PubMed]
Cite this article as: Zamboni S, Lonati C, Palumbo C, Marconi MC, Mondini F, Lattarulo M, Moschini M, Cristinelli L, Belotti S, Simeone C. The effect of perioperative blood transfusion on oncological outcomes in radical cystectomy patients: a narrative review. AME Med J 2020;5:18.

