
Updates in the management of benign and malignant scrotal conditions: issues on surgical ablation and reconstruction
Malignant scrotal conditions
The first and classical description of primary cancer of the scrotum was attributed to Percival Pott which recognized it as an occupational disease in 1775 (1). However, the lesion had been apparently first described by Bassius in 1731 and later by Treyling in 1740 (2). Pott identified it among chimney sweepers and because of this association the tumor has become synonymous with “Pott’s cancer” or “chimney sweeps” cancer. This was the first recognition of an occupationally related malignancy. Since then, this malignancy has also been found in association with coal distillation process, shale oil workers, wax pressman, cotton mule spinners and metal machinists (3-7). In addition, this disease was found to be prevalent among nomadic tribesmen of Persia and Turkistan, who carried charcoal-filled pots under their robes for warmth. As further research was conducted, carcinogenic polycyclic aromatic hydrocarbons in soots, tar, arsenic, paraffin, shale oil, among other have all been linked to the development of scrotal cancer (2,8-10).
Malignant tumors of the scrotum are rare. Despite their low incidence, squamous cell carcinoma (SCC) is the most common scrotal cancer with a propensity for recurrence and distant spread (11,12). It represents one of the most common forms of scrotal malignancy. Improved knowledge on occupational risk factors, decline of then cotton industry and improved work protection and conditions contributed significantly to the falling incidence of this disease (8,13).
Scrotal cancer should not be confused with the more common testicular cancer affecting predominantly young adults. When searching the internet using keywords such as “scrotal cancer” or “male external genitalia cancer”, it is quite common to be supplied with literature on testicular cancer by the internet search engines. The scrotum is the external pouch of skin and muscles containing the testicles. Consequently, scrotal tumors are mostly cutaneous tumors or, more rarely, sarcomas. Although tumors of the scrotal skin might be classified as cutaneous cancer, due to the strong relationship between scrotal tumor and occupational exposures and the lack of this relationship with other skin tumors, scrotal carcinoma is classified as a separate entity.
Limited and sparse information on epidemiology, treatment and outcomes of scrotal cancer has been published in the last three decades, probably due to its low incidence, except, to our knowledge, for three studies with relatively small patient cohorts (11,14,15). Despite improved awareness and elimination of occupational carcinogens over the last century which led to an initial reduction in its incidence, SCC has maintained a steady low incidence rate. The objective of this article is to review and update the recent published literature on scrotal SCC, highlight changes in epidemiology, emerging therapies and surgical reconstruction issues with its impact on quality of life (QoL) of the cancer survivor.
Epidemiology
Although long noted for its historical relevance, scrotal cancer has not received great attention and, therefore, has not been well characterized. Most previous studies have been restricted to case reports and small case series evaluating exposure to environmental risk factors. Sparse studies have dealt with epidemiology of scrotal SCC (16-18). Vyas et al. used two Surveillance Epidemiology and End Results (SEER) database studies, one Netherlands Cancer Registry (NCR) based analysis, one prospective multi-institutional study and two retrospective studies to assess epidemiology (11,12,16,17). Studies from the mid-to-late 1990s reported SCC to account for 80–100% of all scrotal malignancies. However, more recent studies mention this histology is responsible for one third only of all scrotal malignancies (11,12,14,17). Nonetheless, scrotal SCC still remains the most common malignant histology (11,12,17). Other scrotal tumors, like in penile cancer or other skin malignancies, include sarcoma, extramammary Paget’s disease, Bowen’s disease or SCC in situ and adnexal skin tumors. Median age at diagnosis ranges from 52 to 57 years (11,19). It is more common in Caucasians followed by Black and Asian men.
Verhoeven et al. reported the age-standardized incidence rate of scrotal SCC in the Netherlands from 1986 to 2006 and found that it varied between 0.34 and 0.44/1,000,000 male person-years with no statistically significant change over time (11). Wright et al. reported on the age-adjusted incidence rate of scrotal SCC in the United States over a similar period of time and noticed an increase from 0.49/1,000,000 males in 1973 to 0.95/1,000,000 in 2002 (12,20). However, no change in incidence rates by histologic type was no noticed. Moreover, the incidence reported from the Connecticut Tumor Registry data from the period between 1935 and 1979 revealed unchangeable incidence rates of all scrotal malignancies (21). The emergence of new risk factors, such ultraviolet A phototherapy for the treatment of skin diseases (18) and human papilloma virus (HPV) has been the source of speculation for this sustained incidence despite avoidance of known occupational carcinogens.
Survival according to histologic subtypes was studied by Johnson et al. (17). Scrotal SCC, melanoma and adnexal skin tumors were categorized as high-risk scrotal cancer and basal cell carcinoma, Bowen’s disease and sarcoma as low-risk based on median overall survival. Median overall survival for high-risk and low-risk was 118 and 166 months, respectively. Overall survival for scrotal SCC was 115 months (range, 97–133 months). Overall survival for adnexal skin tumors was 114, the lowest survival rate (17). Wright et al. reported statistically significant worse survival for those with SCC compared to those with other histologic types (12). Verhoeven reported 77% 5-year survival for scrotal SCC (11).
Several authors have linked the psoralens and ultraviolet A radiation (PUVA) used for the treatment of psoriasis and other skin diseases with the development of scrotal SCC (22-24). Therefore, genital protection for men undergoing UV radiation for treatment of skin diseases should be recommended.
Matoso et al. found a 24.1% association risk between scrotal SCC and high-risk HPV by in situ hybridization evaluation in a series of 29 patients (19). If the true association of HPV and scrotal SCC is between 24% and 42% as shown in these small series, this has critical implications for preventive therapy through implementation of HPV vaccines.
Chronic mechanical irritation has also been associated with scrotal SCC. These chronic irritants include long-term use of rubber urinals, topical nitrogen mustard and coal tar (25-27). Although Caucasians seem to be more commonly affected, scrotal SCC may affect all other ethnicities (28). An increased risk of a second malignancy, either preceding or following scrotal SCC has been reported (29). This increased risk was later confirmed in a study by Verhoeven where 18% of patients with scrotal malignancy developed other tumors such as lung cancer, skin SCC and second scrotal neoplasm as the most common second malignancy (11).
Risk factors
Although initially thought that SCC was the predominant histologic type of scrotal neoplasm, this concept was challenged by a recent study by Wright, where SCC comprised 32% of scrotal malignancies (12). Case reports have linked scrotal sarcomas with UV radiation, radiotherapy and acquired immunodeficiency syndrome (30,31). In the latter case, Kaposi sarcoma is the usual type.
Race has been also evaluated as risk factor. Historically, overall incidence of SCC of the scrotum remains lower in blacks compared to whites. However, the racial distribution was markedly different in one study, where SCC comprised 35% of malignancies in whites compared with 69% in blacks (12). Several risk factors have been identified:
- Industrial exposure (printing industry, oil workers, steel and metal-related industry);
- Carcinogenic metals including arsenic, nickel, and chromium;
- Iatrogenic (Fowler’s solution, PUVA exposure, XRT exposure);
- Chronic inflammation, poor hygiene and chronic mechanical irritation;
- HPV and HIV infection;
- Radiation exposure;
- Immunosuppression.
Diagnosis
Clinically, SCC of the scrotum presents with a painless erythematous skin lesion that fails to heal. For unknown reason, the left side of the scrotum appears to be preferred (6,15). Diagnosis is biopsy based and the main differential diagnosis is extramammary Paget’s disease as well as verrucous carcinoma and Bowenoid papulosis (32-34).
With a latency ranging from 2 months to 10 years with a mean of 22 months (35), the carcinoma will advance locally and form a painful skin ulcer. The perineal skin can be involved as well as the penile skin. Lymph node metastases develop in the inguinal lymph nodes uni- or bilaterally without predilection of the side of the primary tumor. While being overall rare, distant metastases can happen as early as 4 months after diagnosis or as late as 10 years (36).
Staging
The most commonly used staging system was described by Lowe in 1983 (37) which is based on the extent of the disease locally and of the level of its metastases (Table 1) (37).
Table 1
| A1: disease confined to scrotum |
| A2: locally extensive disease involving adjacent structures (penis, perineum, testis, spermatic cord, and pubic bone) by continuity but without evident metastasis |
| B: metastatic involvement of superficial lymph nodes, amenable to resection |
| C: metastasis to pelvic lymph nodes or any unresectable metastasis |
| D: metastasis to distant organs or beyond regional nodes |
Therefore, similar to penile cancer, clinical staging requires evaluation of the inguinal lymph nodes and pelvic lymph nodes to determine the extent of metastases. Fine needle biopsies have been employed successfully for nodal diagnosis (38) and also 18F-FDG PET/CT appears to have a role in staging (39). Sentinel lymph node biopsy may play a role in high-risk patients (40). Lastly, a plain chest X-ray to exclude pulmonary metastases is recommended.
Management
Primary tumor
The mainstay of treatment is surgical excision with an attempt to achieve a surgical margin. While several centimeters of margin have traditionally been recommended, this concept has been challenged in favor of less extensive resection (41). After excision, the scrotum needs to be reconstructed with the options delineated below.
Inguinal lymph nodes
Neither clinical exam nor imaging can reliably diagnose or exclude metastatic disease (42). Similar to penile cancer, less than 50% of patients with palpable inguinal lymph nodes will have metastatic disease and an automated lymph node dissection has been questioned (36). Recently, a risk-based approach based on sentinel node biopsies has been suggested with completion of the lymph node dissection if this is positive (43).
Locally advanced and metastatic disease
Adjuvant chemotherapy has not been found to influence outcome (32). However, in inoperable disease, combination therapy with methotrexate, bleomycin, and cisplatin is employed with a response rate of 72% (43).
Conclusions
SCC of the scrotum is overall rare while still among the most common scrotal malignancies. Risk factors include, chronic inflammation, chronic mechanical irritation, immunosuppression including HIV, HPV infection, and radiation-exposure. Diagnosis is made via a biopsy and accurate staging requires inguinal and pelvic lymph node evaluation. Surgical resection with scrotal reconstruction remains the preferred treatment followed by a risk-based approach to proceed with inguinal lymph node dissection. For inoperable cases, a cisplatin-based chemotherapy regimen can be employed
Benign scrotal conditions
There are several non-cancerous conditions that are associated with loss of scrotal tissue as well as potentially loss of testicles. Options for scrotal reconstruction are determined by the amount of scrotal skin loss and whether testicles are viable and can be preserved. Over the course of several past decades scrotal reconstructive techniques have progressively emerged and continue to be refined. As will be demonstrated in this chapter, every technique has its advantages and disadvantages and the principle concept of scrotal reconstruction is that one size does not fit all. Therefore, at our institution, scrotal reconstruction is often a combined effort of Urology and Plastic Surgery as the combination of talents and experience is needed. A successful scrotal reconstruction is one that is a durable reconstruction with minimal post-operative wound complications (unfortunately, a certain level of wound complication needs to be expected), a cosmetically appropriate result that satisfies the patient’s expectations, and viable testicles with ideally normal spermatogenesis.
Undoubtedly, the best way to reconstruct a scrotum is with existing scrotal skin (replace like with like). The pliability and elasticity of the scrotal skin with its abundance of rugae as well as the elasticity of the underlying dartos tissue containing the blood supply and lymphatic and venous drainage allows for significant expansion of remaining scrotal tissue. In general, a 60% loss of scrotal skin can be compensated by the remaining 40%. In some cases, successful reconstruction can be achieved with even less remaining tissue. If there is any way that reconstruction can be achieved with existing scrotal skin this should be pursued as all adjuvant techniques will not achieve an equal result.
Simple scrotal reconstruction (Figure 1) with existing tissue follows general rules of reconstructive surgery. The tissue to be covered should be free of infection and bleeding. The existing scrotal skin is stretched to cover the scrotal contents. If needed, the skin can be reconfigured with Z-plasty incisions to gain additional length and/or width. A multi-layer closure of the dartos tissue with absorbable sutures such as 3-0 PDS should be perform to divide tissue tension among multiple sutures. The skin can be closed with interrupted absorbable sutures such as 3-0 Chromic.
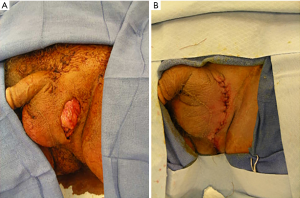
The remainder of this chapter will focus on adjuvant techniques that become necessary if there is insufficient or no existing scrotal skin left. While the scrotal reconstruction of any condition associated with significant scrotal skin loss is identical, some conditions should be highlighted as they come with specific implications for reconstruction.
Hydradenitis suppurativa
HS is a chronic skin condition that is associated with inflamed and swollen skin lumps and is estimated to affect 1–4% of the population with a higher incidence in females than in males (44). Areas affected are the axilla, groin, perianal and perineal areas also involving the scrotum (Figures 2 and 3). The exact etiology is unclear but the lumps are believed to be an inflammatory condition of apocrine sweat glands. There appears to be both a genetic and environmental component as a third of patients also have affected family members (45) while obesity and smoking is present frequently in affected patients. Whereas milder cases can be treated conservatively, the treatment for severe cases is wide surgical excision of the affected area which often involves removal of large parts of the scrotum.
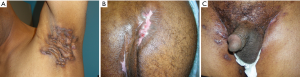
Necrotizing fasciitis (NF) (Fournier’s gangrene)
NF is a rapidly progressive infectious condition of the genital, groin, and perineal area that has historically been associated with a high rate of mortality due to septic shock and multi-organ failure. In contemporary series this is far lower likely due to improved access to emergency medical services and early treatment initiation with antibiotics and surgical debridement (46). NF usually affects patients with an immunocompromised state such as poorly controlled diabetes, HIV infection, chronic alcohol abuse, blood dyscrasias, iatrogenic immunosuppression (e.g., transplant patients). Entry for bacteria is often a small skin lesion allowing the bacteria to proliferate within the subdermal soft tissue. Release of toxin leading to further tissue breakdown facilitates the spread often leading to a sizeable affected area. Hallmark of diagnosis is crepitus noticed on physical exam, however, the presence of cellulitis in the setting of clinical signs such as rapid deterioration, leukocytosis (albeit leukopenia can be present depending on the underlying etiology of immunocompromise), fever, elevated C-reactive protein and platelets should raise suspicion for NF. Treatment of NF is emergent surgical debridement of all affected necrotizing tissue to the level of healthy tissue. The wound is left open and covered with wet-to-dry dressing. A second and even third or more additional visits to the operating room is necessary to fully debride any further necrotized tissue. Only once the wound has stabilized and granulation tissue has developed surgical reconstruction of the skin including scrotal defect can be attempted. Unfortunately, NF is often associated with large skin defect often involving scrotal skin that make this etiology a challenging entity for reconstruction.
Scrotal trauma and burn wounds
The vast majority of scrotal trauma fall into one of the following categories: blunt or penetrating trauma, burn wounds, or animal bites.
Penetrating wounds rarely cause extensive genital skin damage that requires elaborate reconstruction. They can usually be treated at time of exploration or even at bedside with a simple closure if no further exploration is required.
Burns of the genitalia are rare (47) and the severity dictates the reconstructive management with extensive third-degree burns with skin necrosis being the only indication once debridement of necrotic tissue has been completed which may require multiple trips to the operating room.
Animal (and human) bite wounds are characterized by wound infection by microbial flora resident in the oral cavity of the animal which is substantially different from commonly encountered infections of other etiologies and which remains unknown when the patients presents to seek medical care. While small bite wounds can be treated with wound cleaning and closure, extensive injuries require initial conservative treatment with broad spectrum antibiotic and debridement of necrotic tissue. Only once the wound remains without signs of infection—which usually is 48 hours (48)—reconstruction can be performed.
Scrotal lymphedema (SL)
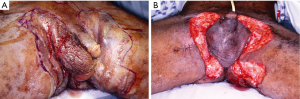
SL develops as sequelae for a variety of underlying conditions including pelvic radiation or surgery, infection, neoplasm (49) and can affect the scrotum in an isolated manner or it can be associated with for example bilateral leg edema (Figure 4). Addressing the underlying pathology is a necessary initial step and often this as well as conservative therapy with scrotal elevation is sufficient treatment (49). The treatment of chronic LS consists of excision of the edematous skin with its dilated subcutaneous lymphatic channels. Commonly, the chronic lymphedema with its decreased ability to supply adequate perfusion to the skin combined with elevated pressures will lead to skin damage which makes it unsuitable for its use in reconstruction. After complete resection and having addressed a potential underlying condition scrotal reconstruction using one or more of the techniques described below can be performed.
Morbid obesity
A condition that is more and more frequently encountered in scrotal reconstruction are previously morbidly obese patients that lost weight and are now confronted with large amounts of excessive skin (Figure 5). Scrotal reconstruction along with concomitant penile unburying and concomitant or staged panniculectomy is the treatment of choice and often necessary as the scrotum can extend very far inferiorly causing a myriad of problems including for example inability to walk. While often not all skin is usable, specifically that of a previously buried scrotum or penile shaft skin, there often is sufficient skin for grafts or flaps present due to the amount of excess skin.
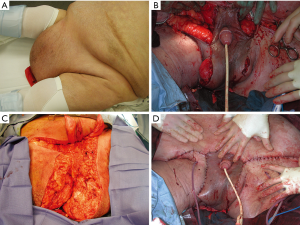
Scrotal reconstructive techniques
The main techniques for scrotal reconstructing encompass skin grafts (full thickness or split thickness) and fasciocutaneous and myocutaneous flaps. The main challenge with reconstruction of the scrotum with any of these techniques is the mobility of the scrotum itself and of its contents which complicate any techniques. We recommend that for every reconstructive effort, especially when pursuing skin grafting, the testicles should be attached to each other with an absorbable suture to prevent torsion and to decrease mobility. When confronted with a difficult repair, the placement of one or often both testes in thigh pouches is an important adjunct maneuver that should be considered as it facilitates the reconstruction leading to increased success of grafts and flaps.
Other considerations include:
- Infectious and traumatic etiology of scrotal skin loss: has all infected or necrotic tissue been removed and is the tissue receptive for reconstructive efforts (e.g., granulation tissue has formed)?
- Lymphedematous etiology: has the underlying pathology be adequately addressed?
- Morbidly obese patients: has the weight loss been sufficient and/or is further weight loss expected (in which case the reconstruction should be postponed until this has been achieved)?
- The presence of viable testicles: if necrosis of the testis is suspected a doppler ultrasound can confirm non-viability and a uni- or bilateral orchiectomy should be performed
- What are the patient goals in regards to fertility?
- What is the surgeon’s experience with reconstructive techniques? As mentioned above, in most cases it is advantageous to perform the reconstruction in conjunction with a plastic surgeon
Skin grafts
Split thickness skin grafts (STSG) are commonly used for scrotal skin reconstruction. Skin can be harvested from the upper thigh or the lower abdomen and in cases of reconstruction of a morbidly obese patient ALSO from the removed excess tissue. The skin is harvested with a dermatome at a thickness of 0.018 inches followed by meshing which increases the surface areas. Besides needing less tissue to cover more area, the advantage of a STSG over a full thickness skin graft (FTSG) is that it allows drainage of serous fluid that accumulates underneath the graft and it is less prone to scar contracture. The lack of subcutaneous tissue including lymphatic vessels makes this a preferred skin graft for patients with lymphedema as etiology as it decreases the risk of recurrence (50). Being a thin cover, STSG also helps keep the temperature within the neoscrotum low improving spermatogenesis (51). The use of a fibrin sealant such as Artiss or Tisseel that is sprayed on the receptive site facilitates a close contact of the graft to the underlying tissue and decreases its motility which subsequently improves imbibition and inosculation leading to improved graft survival. The graft is then sutured to the underlying tissue at its edges as well as quilted in abundant places to minimize shearing.
FTSG are more prone to contraction. While this poses less of a problem in scrotal reconstruction than for example in penile skin reconstruction, this needs to be considered when the graft is outlined prior to harvest to yield sufficient tissue. As large defects will need a large skin graft it can be difficult to find an appropriate harvest site. One advantage of FTSG is that it is more resistant to mechanical trauma than STSG which allows for less concerns regarding the patient’s mobility post-operatively. Presence of hair follicles may also be desired to contribute to a more natural looking scrotum. As this graft is thicker and more tissue needs to be supported by the recipient site it is important to remove any excess subcutaneous adipose tissue to improve graft survival. FTSG should be pie crusted to allow for drainage of post-operative fluid accumulating underneath the graft. As in STSGs, a fibrin glue can be used to improve adherence and graft survival. The graft is then sewn to the edges of the recipient tissue and quilted in several locations.
Skin flaps
Unlike grafts flaps do not have to rely on vascular ingrowth for their viability as they bring the blood supply with them. Also, innervation is preserved which allows for sensitivity in the recipient area. Main disadvantages of flaps are the morbidity associated with harvest including the creation of a second wound.
Reconstruction using adjacent skin
The skin adjacent to the defect can be a good choice to use for scrotal reconstruction when the defect is small enough. Most commonly a simple V-Y advancement, rotation or transposition can be used.
Local skin flaps
Local cutaneous flaps are also useful for small to medium -sized defects, they can be brought into the scrotal defect with rotation, advancement, or transposition. Blood supply to the scrotum is provided by the inferior external pudendal artery laterally and two scrotal arteries branching from the perineal arteries centrally (52) and flaps based on these vascular supplies can be isolated and brought into the defect. Once the flap has been brought into the desired position, it is sutured in place both at the edges as well as its underside.
Fasciocutaneous and musculocutaneous flaps
Both fasciocutaneous and musculocutaneous flaps can be used for scrotal reconstruction. Preservation of underlying tissue (subcutaneous fascia and subcutaneous fascia and muscle) allow for a robust blood supply. This advantage is important specifically for wounds in which the existing blood supply is tedious. Although the bulkiness is often seen as disadvantage especially for the cosmetic appearance of the neoscrotum, it is helpful when deeper defects have to be filled, for example after tissue resection for NF. Selection of proceeding with either fasciocutaneous and musculocutaneous should therefore incorporate depth of recipient tissue bed. Possible unwanted bulkiness, especially when using musculocutaneous flaps, can however also be concerning.
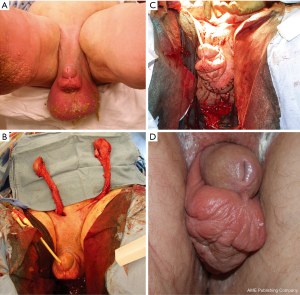
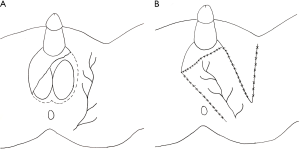
A number of fasciocutaneous flaps have been reported in the literature such as superomedial thigh flaps (53,54), anteromedial/medial thigh flaps (55), anterolateral thigh flaps, or pudendal thigh flaps (56) which is illustrated in Figure 6. There is evidence that spermatogenesis may be preserved in testicles covered with a fasciocutaneous flap, specifically if adequately thinned (57).
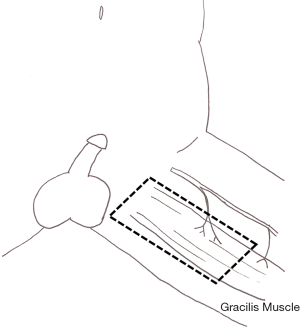
Musculocutaneous flaps are less frequently used unless the tissue defect is too deep for a fasciocutaneous flap. Their bulkiness otherwise often leads to cosmetically undesired outcomes and they also lack the thermoregulatory advantages of fasciocutaneous flaps with expected decreased spermatogenesis. Musculocutaneous flaps reported in the literature include a short gracilis flap (58) which can be used uni- or bilaterally (59) and which is illustrated in Figure 7.
Thigh pouches
Placing testicles in thigh pouches can be a temporary maneuver to allow the remaining scrotal tissue to heal prior to reconstruction. It alleviates the need to wrap testicle in gauze while granulation tissue develops in preparation for definitive reconstruction. In addition, it prevents granulation tissue to form on the testicles and the cord. This thick rind needs to be removed prior to reconstruction not only to regain mobility of cord and testes but also for the thickness of it and its chronically infected state.
However, thigh pouches can be also part of definitive when scrotal reconstruction is not possible without placing the testicles outside the area to be reconstructed. We believe this is an important adjunct maneuver to consider in cases when scrotal reconstruction is most tedious. Criticism of thigh pouches include patient dissatisfaction as the testicle located in the superior aspect of the medial thigh are prone to mechanical irritation and thus causing pain. Also, intratesticular temperature is elevated and spermatogenesis affected. However, the temperature can be considered lower than when covered by a musculocutaneous flap.
Thigh pouches are generated bluntly superior to the fascia lata and should be located as caudal and as posterior as possible to avoid stretching of the cord when the thighs are abducted. Access is gained via the genital tissue defect, a Deaver retractor can help identify the fascia lata. A ring forceps can then be inserted and carefully opened to generate a sufficiently large subcutaneous space to receive the testicle. Both cords are mobilized to the external inguinal ring and then the testicle placed into the pouch. If definitive thigh pouch placement is performed, a counterincision over the caudal aspect of the thigh pouch can be made and the tunica albuginea of the testicle sutured to the fasica lata with non-absorbable sutures.
Summary
A number of malignant and benign conditions can lead to significant genital skin loss. Due to the constant mobility and mechanical stress (e.g., walking, sitting) that a scrotum experiences, the outcome of grafts and flaps are not comparable to those at other body sites. The ideal tissue for scrotal reconstruction is the remaining scrotal tissue and only if >60% of it is lost additional maneuvers need to be performed. The reconfiguring of adjacent skin tissue to cover the defect is preferred as next best option. Skin grafts, preferably split thickness, is an option if the defect is insufficiently covered and this can be combined also with fasciocutaneous (or less frequently musculocutaneous) flaps which, however, experience significant strain and less optimal outcome given the mobility and mechanical stress. Thigh pouches can be a valid option, both temporary and definitive, and should be part of the reconstructive armamentarium.
Acknowledgments
The authors thank Dusty Williams-Hofer for the illustrations in Figures 6 and 7.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Philippe E. Spiess) for the series “Rare Genitourinary Malignancies” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj.2020.03.08/coif). The series “Rare Genitourinary Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pott P. Cancer Scroti. In: Hayes L, Clarke W, Collins R. editors. Chirurgical Works. London: Longman, 1775:5p. 63.
- Graves RC, Flo S. Carcinoma of the scrotum. J Urol 1940;43:309. [Crossref]
- Waldron HA. A brief history of scrotal cancer. Br J Ind Med 1983;40:390-401. [PubMed]
- Hendricks NV, Berry CM, Lione JG, et al. Cancer of the scrotum in wax pressmen. AMA Arch Ind health 1959;19:524-9. [PubMed]
- Cruickshank CND, Squire JR. Skin cancer in the engineering industry from the use of mineral oil. Br J Ind Med 1950;7:1-11. [PubMed]
- Southam AH, Wilson SR. Cancer of the scrotum; the etiology, clinical features and treatment of the disease. Br Med J 1922;2:971-970.1. [Crossref] [PubMed]
- Fife JG. Carcinoma of the skin in machine setters. Br J Ind Med 1962;19:123-5. [PubMed]
- Lee WR, McCann JK. Mule spinners’ cancer and the wool industry. Br J Ind Med 1967;24:148-51. [PubMed]
- Dean AL. Epithelioma of scrotum. J Urol 1948;60:508-18. [Crossref] [PubMed]
- Kipling MD, Waldron HA. Polycyclic aromatic hydrocarbons in mineral oil, tar and pitch, excluding petroleum pitch. Prev Med 1976;5:262-78. [Crossref] [PubMed]
- Verhoeven RH, Louwman WJ, Koldewijn EL, et al. Scrotal cancer: incidence, survival and second primary tumours in the Netherlands since 1989. Br J Cancer 2010;103:1462-6. [Crossref] [PubMed]
- Wright JL, Morgan TM, Lin DW. Primary scrotal cancer: disease characteristics and increasing incidence. Urology 2008;72:1139-43. [Crossref] [PubMed]
- Lowe FC. Squamous-cell carcinoma of the scrotum. Urol Clin North Am 1992;19:397-405. [PubMed]
- Kickham CJE, Dufresne M. An assessment of carcinoma of the scrotum. J Urol 1967;98:108-10. [Crossref] [PubMed]
- McDonald MW. Carcinoma of scrotum. Urology 1982;19:269-74. [Crossref] [PubMed]
- Vyas R, Homayoun Z, Trolio RD, et al. Squamous cell carcinoma of the scrotum: a look beyond the chimneystacks. World J Clin Cases 2014;2:654-60. [Crossref] [PubMed]
- Johnson TV, Hsiao W, Delman KA, et al. Scrotal cancer survival is influenced by histology: a SEER study. World J Urol 2013;31:585-90. [Crossref] [PubMed]
- Stern RS, Bagheri S, Nichols K. The persistent risk of genital tumors among men treated with psoralen plus ultraviolet A (PUVA) for psoriasis. J Am Acad Dermatol 2002;47:33-9. [Crossref] [PubMed]
- Matoso A, Ross HM, Chen S, et al. Squamous neoplasia of the scrotum: a series of 29 cases. Am J Surg Pathol 2014;38:973-81. [Crossref] [PubMed]
- Parys BT, Hutton JL. Fifteen-year experience of carcinoma of the scrotum. Br J Urol 1991;68:414-7. [Crossref] [PubMed]
- Roush GC, Schymura MJ, Flannery JT. Secular and age distribution of scrotal cancer in Connecticut and a review of United States literature. Cancer 1984;54:596-601. [Crossref] [PubMed]
- Taniguchi S, Furukawa M, Kutsuna H, et al. Squamous cell carcinoma of the scrotum. Dermatology 1996;193:253-254. [Crossref] [PubMed]
- Loughlin KR. Psoriasis: association with 2 rare cutaneous urological malignancies. J Urol 1997;157:622-3. [Crossref] [PubMed]
- Stern RS. Genital tumors among men with psoriasis exposed to psoralens and ultraviolet A radiation (PUVA) and ultraviolet B radiation. The Photochemotherapy Follow-up Study. New Engl J Med 1990;322:1093-7. [Crossref] [PubMed]
- Grundy D, Jones AC, Powley PH. Carcinoma of the scrotum associated with rubber urinals. Case report. Paraplegia 1993;31:616-7. [PubMed]
- Lee LA, Fritz KA, Golitz L, et al. Second cutaneous malignancies in patients with mycosis fungoides treated with topical nitrogen mustard. J Am Acad Dermatol 1982;7:590-8. [Crossref] [PubMed]
- Moy LS, Chalet M, Lowe NJ. Scrotal squamous cell carcinoma in a psoriatic patient with coal tar. J Am Acad Dermatol 1986;14:518-9. [Crossref] [PubMed]
- Oluwasanmi JO, Williams AO, Alli AF. Superficial cancer in Nigeria. Br J Cancer 1969;23:714-28. [Crossref] [PubMed]
- Gerber WL. Scrotal malignancies; the University of Iowa experience and a review of the literature. Urology 1985;26:337-42. [Crossref] [PubMed]
- Good J, Cooper S. The Study of Medicine. Thoma and Geroge Underwood, 1829:313-5.
- Vapnek JM, Quivey J, Carroll P. Acquired immunodeficiency syndrome-related Kaposi’s sarcoma of the male genitalia: Management with radiation therapy. J Urol 1991;146:333-6. [Crossref] [PubMed]
- Miyakawa T, Togawa Y, Matsusima H, et al. Squamous metaplasia of Paget’s disease. Clin Exp Dermatol 2004;29:71-3. [Crossref] [PubMed]
- Du X, Yin X, Zhou N, et al. Extramammary Paget’s disease mimicking acantholythic squamous cell carcinoma in situ: a case report. J Cutan Pathol 2010;37:683-6. [Crossref] [PubMed]
- Criton S, Aravindam KP. Bowenoid papulosis turning to squamous cell carcinoma Indian J Dermatol Venereol Leprol 1998;64:37-9. [PubMed]
- Andrews PE, Farrow GM, Oesterling JE. Squamous cell carcinoma of the scrotum: long-term follow-up of 14 patients. J Urol 1991;146:1299-304. [Crossref] [PubMed]
- Ray B, Whitmore WF Jr. Experience with carcinoma of the scrotum. J Urol 1977;117:741-5. [Crossref] [PubMed]
- Lowe FC. Squamous cell carcinoma of the scrotum. J Urol 1983;130:423-7. [Crossref] [PubMed]
- Djajadiningrat RS, Teertstra HJ, van Werkhoven E, et al. Ultrasound examination and fine needle aspiration cytology useful for follow-up of the regional nodes in penile cancer? J Urol 2014;191:652-5. [Crossref] [PubMed]
- Rosevear HM, Williams H, Collins M, et al. Utility of 18F-FDG PET/CT in identifying penile squamous cell carcinoma metastatic lymph node. Urol Oncol 2012;30:723-6. [Crossref] [PubMed]
- Greene TD, Abrahams HM, Moran ME. Dynamic lymphoscintigraphy for stating scrotal carcinoma. J Urol 2001;165:536-7. [Crossref] [PubMed]
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU int 2005;96:1040-3. [Crossref] [PubMed]
- Hughes B, Leijte J, Shabbir M, et al. Non-invasive and minimally invasive staging of regional lymph nodes in penile cancer. World J Urol 2009;27:197-203. [Crossref] [PubMed]
- Lowe FC. Squamous cell carcinoma of the scrotum. Urol Clin North Am 1992;19:397-405. [PubMed]
- Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med 2012;366:158-64. [Crossref] [PubMed]
- Sartorius K, Lapins J, Emtestam L, et al. Suggestions for uniform outcome variables when reporting treatment effects in hidradenitis suppurativa. Br J Dermatol 2003;149:211-3. [Crossref] [PubMed]
- Sorensen MD, Krieger JN. Fournier's Gangrene: Epidemiology and Outcomes in the General US Population. Urol Int 2016;97:249-59. [Crossref] [PubMed]
- Michielsen D, Van Hee R, Neetens C, et al. Burns to the genitalia and the perineum. J Urol 1998;159:418-9. [Crossref] [PubMed]
- van der Horst C, Martinez Portillo FJ, Seif C, et al. Male genital injury: diagnostics and treatment. BJU Int 2004;93:927-30. [Crossref] [PubMed]
- Garaffa G, Christopher N, Ralph DJ. The management of genital lymphoedema. BJU Int 2008;102:480-4. [Crossref] [PubMed]
- Malloy TR, Wein AJ, Gross P. Scrotal and penile lymphedema: surgical considerations and management. J Urol 1983;130:263-5. [Crossref] [PubMed]
- Wang DL, Luo ZJ, Sun GF, et al. Long-term prognosis of free skin-grafted penoscrotal avulsion injuries in two patients. J Plast Reconstr Aesthet Surg 2009;62:385-7. [Crossref] [PubMed]
- Carrera A, Gil-Vernet A, Forcada P, et al. Arteries of the scrotum: a microvascular study and its application to urethral reconstruction with scrotal flaps. BJU Int 2009;103:820-4. [Crossref] [PubMed]
- Oufkir AA, Tazi MF, El Alami MN. The superomedial thigh flap in scrotal reconstruction:Technical steps to improve cosmetic results. Indian J Urol 2013;29:360-2. [Crossref] [PubMed]
- Hirshowitz B, Moscona R, Kaufman T, et al. One-stage reconstruction of the scrotum following Fournier's syndrome using a probable arterial flap. Plast Reconstr Surg 1980;66:608-12. [Crossref] [PubMed]
- Maguina P, Paulius KL, Kale S, et al. Medial thigh fasciocutaneous flaps for reconstruction of the scrotum following Fournier gangrene. Plast Reconstr Surg 2010;125:28e-30e. [Crossref] [PubMed]
- Karaçal N, Livaoglu M, Kutlu N, et al. Scrotum reconstruction with neurovascular pedicled pudendal thigh flaps. Urology 2007;70:170-2. [Crossref] [PubMed]
- Wang D, Wei Z, Sun G, et al. Thin-trimming of the scrotal reconstruction flap: long-term follow-up shows reversal of spermatogenesis arrest. J Plast Reconstr Aesthet Surg 2009;62:e455-6. [Crossref] [PubMed]
- Kayikçioğlu A. A new technique in scrotal reconstruction: short gracilis flap. Urology 2003;61:1254-6. [Crossref] [PubMed]
- Noack N, Spierer R. Uni- and bilateral pedicled muscular gracilis flaps for reconstruction of the scrotum in Fournier's gangrene. Handchir Mikrochir Plast Chir 2009;41:248-51. [Crossref] [PubMed]
Cite this article as: Hofer MD, Dumanian GA, Felício J, Martins FE. Updates in the management of benign and malignant scrotal conditions: issues on surgical ablation and reconstruction. AME Med J 2020;5:27.

