
Conventional coronary artery bypass grafting
Introduction
An old-fashioned conventional coronary artery bypass grafting (CABG) procedure involves cutting the skin through the subcutaneous tissues down to the sternum, accessing the heart through a full median sternotomy, harvesting the left internal mammary artery (LIMA) while saphenous vein grafts (SVGs) being simultaneously harvested, vertically dividing the pericardium, thymic tissue remnant and pericardial fat in the midline, cannulating the aorta and the right atrium, cross-clamping the aorta, arresting the heart via antegrade cold blood cardioplegia, performing distal and proximal anastomoses (1). But is this the whole truth? Is conventional always old-fashioned? Is it less than an optimal option to perform CABG currently?
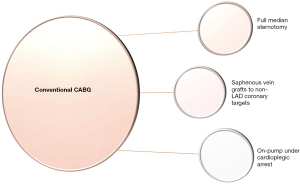
During the current era of domination of percutaneous coronary intervention (PCI) with drug-eluting stents (DES), coronary artery bypass grafting (CABG) continues to be the gold standard means of revascularization in multivessel coronary artery disease (CAD) (1,2). It was in 1969 that Dr René Favaloro first used an SVG the way we use it today to perform an aorto-coronary bypass (3). Since then, there have been plenty of trials in the literature trying to prove the inferiority or not of conventional CABG. But how is conventional CABG defined? Full median sternotomy, LIMA to left anterior descending coronary artery (LAD) anastomosis and SVGs to other coronary artery targets under the safe, motionless and bloodless environment of cardiopulmonary bypass under cardioplegic arrest is typical of conventional CABG (1) (Figure 1). Then what is the non-conventional choice? Bilateral internal mammary artery (BIMA) harvesting or total arterial revascularization (TAR) instead of SVGs, off-pump CABG instead of on-pump CABG under cardioplegic arrest and minimally invasive direct coronary artery bypass (MIDCAB) or hybrid coronary revascularization (HCR) instead of full median sternotomy are the non-conventional options. The value of each one of these aforementioned non-conventional techniques is measured by comparing their results to those of conventional CABG (4,5).
Historical insight and current guidelines on myocardial revascularization
From Alexis Carrel who was awarded with the Nobel Prize in Physiology in 1912 for pioneering vascular suturing techniques (6) to direct suturing of LIMA on the anterior epicardial surface of the heart by Arthur Vineberg in late 1940s (7-9), we entered the era when Sabiston performed the first CABG using an SVG in 1962 (10). However, in 1967 (reported in 1969), René Favaloro was the first surgeon who used an SVG as an interposition graft placed between the ascending aorta and the right coronary artery distal to the blockage, just the way we use it nowadays (11-13). Two years later W. Dudley Johnson and coworkers reported their series consisting of 301 CABG cases performed over a period of 19 months (1) and the history of conventional CABG was well on its way.
According to 2018 ESC/EACTS guidelines on myocardial revascularization (2) critical major coronary artery vessel stenosis over 90%, coronary stenosis over 50% with documented ischemia or fractional flow reserve (FFR) less than 0.8 independently of left ventricular function when left main and proximal LAD lesions are concerned, or accompanied with left ventricular ejection fraction (LVEF) less than 35% in case of other two- or three-vessel disease and over 10% of left ventricle area of ischemia by functional testing are indications for revascularization in case of stable angina or silent ischemia. As far as the question of CABG or PCI is concerned, PCI should not be performed in left main CAD with high SYNTAX score (>33) and in three-vessel CAD with more than intermediate SYNTAX score (>22), whereas CABG is a clearly better choice than PCI in case of three-vessel disease with diabetes mellitus even with low SYNTAX score (0–22) and in left main CAD with >22 SYNTAX score. On the contrary, PCI is a superior choice in case of one- or two-vessel disease without proximal LAD lesion. Finally, CABG and PCI are equal choices in every other case of one-, two-, or three-vessel CAD (2).
Technical experience is critical for performing CABG surgery. Various studies have reported a significant impact of the volume of CABG surgery per hospital, as well as per surgeon, on in-hospital mortality (14-16). Therefore, 2018 ESC/EACTS guidelines on myocardial revascularization suggest that CABG should be performed in institutions performing over 200 CABG cases annually by surgeons having performed over 200 CABG cases under supervision during their training (2). Interestingly, the cut-off value for performing off-pump CABG (OPCAB), which is more technically demanding for the surgeon, was estimated at 50 cases per year for the largest improvement and above 150 cases per year for the lower mortality to be achieved (17).
BIMA or conventional CABG?
Although BIMA grafting is recommended in recent European guidelines on myocardial revascularization (2), its current adoption in clinical practice is limited, as there are thoughts about rendering a CABG operation more complex and because of being accompanied by higher deep sternal wound infection (DSWI) rates. Moreover, its long-term survival benefit is doubted (2,18-22). Although superiority of BIMA grafting in terms of long-term survival compared to single internal mammary artery (SIMA) grafting has been reported by pooled observational studies (19,23), thanks to the excellent long-term angiographic patency of the right internal mammary artery (RIMA) (24,25), the Arterial Revascularization Trial (ART)—the biggest randomized trial comparing BIMA to SIMA grafting—revealed no benefit in terms of long-term survival (26). ART compared 1,548 patients with multivessel CAD randomly assigned to BIMA CABG to 1,554 patients with multivessel CAD offered SIMA CABG (26). The interim analysis of the results at 5 years reported no significant difference regarding all-cause mortality, myocardial infarction, or stroke (27) and everyone waited for the final 10-year results. However, no significant superiority was either revealed in terms of all-cause mortality, the composite outcome of death, myocardial infarction, or stroke and repeat revascularization after BIMA grafting. Results remained similar after adjustment for age, sex, diabetes status and ejection fraction. Neither the rate of early major bleeding events differed between the two groups. Moreover, BIMA grafting was associated with a higher incidence of sternal wound complications (3.5%) compared to SIMA group (1.9%) (26).
DSWI, occurring after 1% to 4% of CABG operations, is related to a remarkably high mortality rate of 25% (28). Although BIMA grafting is a risk factor for sternal wound complications, especially in diabetic patients (29), skeletonization of the mammary grafts partly mitigates the hazard of mediastinitis (30). However, obesity and diabetes are strong independent predictors of mediastinitis (31-33). Therefore, according to the 2018 European guidelines, there is skepticism concerning BIMA grafting in obese patients, those with diabetes mellitus, chronic pulmonary obstructive disease or previous mediastinal radiation. The more of these factors are present, the higher is the possibility of DSWI (26,27,34-36).
TAR OR conventional CABG?
Long saphenous vein in addition to LIMA is the most widely second-choice graft used in the vast majority of patients in North America and Europe (37). Although observational studies reported a clinical benefit from multiple arterial grafts use, randomized clinical trials did not confirm the latter. Therefore, TAR has not gained most surgeons’ trust (38). Arterial grafts such as gastroepiploic artery and others have been described. However, radial artery grafts are most widely utilized when TAR is performed.
Several trials have detected angiographic patency superiority of radial artery grafts (RA) against SVGs (38). Moreover, the most complete retrospective multicenter analysis comparing TAR to conventional CABG in 384 propensity matched pairs of patients, revealed a statistically significant superiority in terms of 15-year survival (54% vs. 41% respectively) (39). However, no significant survival benefit related to the aforementioned superior patency rates was reported by a patient-level combined analysis of randomized, controlled trials performed by the RADIAL investigators (38,40). Six randomized trials comparing clinical outcomes between 534 patients who received RA and 502 patients who received SVGs were analyzed. Although significantly lower rate of the composite primary outcome of death, myocardial infarction, or repeat revascularization at mean follow-up of 50 months was detected in the former group, there was no difference in all-cause mortality (40).
However, various factors may impact RA graft patency. Target vessel size and its runoff and severity of stenosis with the subsequent possibility of competitive flow are some of them. Coronary vessel stenosis less than 70% has a negative impact on RA patency (41,42). In the prospective, randomized Radial Artery Patency Study (RAPS), stenosis over 90% was related to 5.9% occlusion rate which was significantly lower than the 11.8% occlusion rate when the stenosis was 70–89% (43). Moreover, grafting the right coronary bed is related to higher graft failure rate (44-46). RAPS prospective randomized trial reported better 1-year patency of RA (92%) compared to SVGs (86%) (43). However, each patient received both an RA and an SVG in different coronary targets which may have affected the final result. Indeed, a later prospective randomized trial comparing angiograms 10 to 14 months postoperatively between 212 patients who received an RA and 203 patients who received SVGs, and angiograms after 14 months postoperatively between 37 RA patients and 47 SVG patients, revealed no significant difference in 1-year graft patency between the compared groups (42). Interestingly, RA had significantly lower patency rates than SVGs in diabetic patients. Significant differences favoring SVGs were also reported with regard to incidence of 99% occlusion, as well as severe stenosis (75–100%), whereas no significant difference in terms of adverse events and deaths were reported. The difference maker from RAPS study was that each patient received only one study graft, either an RA or SVGs (42).
OPCAB or conventional CABG?
The association of cardiopulmonary bypass with systemic inflammatory response, release of cytokines, activation of the clotting cascade, metabolic disturbances and microembolization led to the evolution of OPCAB. However, initial enthusiasm based on the aforementioned rationale was not translated to better clinical outcomes. No significant difference in 30-day or 1-year clinical results comparing on- with off-pump CABG performed by experienced surgeons were detected by two large, international randomized trials (47-49). Moreover, on-pump CABG (ONCAB) is related to excellent short- and long-term outcomes (5,49-52).
The first large, multicenter, randomized trial examining OPCAB against ONCAB was ROOBY. ROOBY included 2203 patients at Veterans Affairs Centers (5). This trial revealed the superiority of ONCAB as the composite endpoint of mortality, myocardial infarction and repeat revascularization at 1 year was significantly better after ONCAB (9.9% vs. 7.4%, respectively) (5). CORONARY and GOPCABE were subsequent large, randomized trials conducted to answer the question of superiority OPCAB or ONCAB. The CORONARY trial (48,49), the largest randomized trial to date, included 4,752 high-risk patients randomized to OPCAB or ONCAB. No significant differences in terms of 30-day (9.8% vs. 10.3%, P=0.59) or 1-year (12.1% vs. 13.3%, P=0.24) composite primary endpoint of mortality, myocardial infarction and stroke were detected between the two groups. However, patients with higher EuroSCORE derived some benefit from OPCAB (48,49). Similar results showing no difference in terms of composite primary endpoint of death, stroke, myocardial infarction, repeat revascularization or new renal replacement therapy at 30 days (7.8% vs. 8.2%, P=0.74) and at 1 year (13.1% vs. 14.0%, P=0.48) were reported by GOPCABE which randomized 2539 high-risk patients, older than 75 years old to OPCAB or ONCAB (47). Three-year survival rates were also comparable between OPCAB and ONCAB patients in a large observational study by Hannan et al. (52).
Optimal outcomes and durability of CABG are largely affected by completeness of revascularization (53,54). Therefore, completeness of revascularization should not be compromised by the choice of OPCAB. According to ROOBY trial, more patients had fewer than initially planned grafts completed after OPCAB compared to ONCAB (17.8% vs. 11.1% respectively) (5), with higher incomplete revascularization rates reported in the OPCAB group (17.9% vs. 11.1%; P<0.0001). Similarly, inferior rates concerning complete revascularization were reported for the OPCAB group (11.8% vs. 10%, P=0.05 respectively) in the CORONARY trial (48,49), as well as in the GOPCABE trial (34.0% vs. 29.3%) (47). However, whether this ONCAB superiority has an impact on long-term outcomes is debatable. A post-hoc analysis of the ART to assess 5-year outcomes comparing 1260 patients who underwent OPCAB versus 1700 patients who underwent ONCAB was conducted. Five-year mortality and major cardiac and cerebrovascular event (MACCE) risk did not significantly differ between the two groups (55). Similar 5-year outcomes were also reported by the CORONARY trial (56). Furthermore, Takagi et al. (57) conducted a meta-analysis including 5 randomized trials (1,480 patients) and 17 adjusted observational studies (102,820 patients) to explore long-term all-cause mortality after OPCAB or ONCAB. Increased long-term mortality associated with OPCAB was revealed in observational studies, but this was not confirmed by randomized trials showing similar results (57). Moreover, a single-centre retrospective analysis comparing 5,995 OPCAB and 4,875 ONCAB procedures showed no impact of choice of approach on risk of stroke, postoperative haemofiltration, late survival (median follow-up of 12 years) or reintervention (58).
Emergent conversion from OPCAB to ONCAB is another fear related to OPCAB. A meta-analysis of 14 randomized controlled trials reported an incidence of off-pump to on-pump conversion rate from 0% to 13.3%. Surgeons were mostly forced to convert due to haemodynamic instability and the presence of intramyocardial-coronary vessel course. The most-experienced were the surgeons, the less was the conversion rate (59). Increased mortality rates ranging from 6% to 15% are reported after such an emergent on-pump conversion (60-64). The ROOBY trial reported a 12.4% conversion rate (5). These patients had significantly poorer 1-year composite outcome of all-cause mortality, myocardial infarction and revascularization (5). Interestingly, elective conversion to ONCAB does not have an impact on adverse outcomes. The aforementioned post-hoc analysis of the ART trial detected a 2.3% conversion rate and this conversion was associated with remarkably higher in-hospital mortality rates compared to cases without conversion (10.3% vs. 0.7% respectively; P<0.001). There was also a persistent trend of worse outcomes after conversion at 5 years follow-up (55).
On the contrary, there is evidence that OPCAB can be advantageous for high-risk patients. High-risk patients refer to women (65), patients with left ventricular dysfunction (66,67), ST-elevation myocardial infarction (68), prior stroke (69), advanced age (70), renal insufficiency (71-73), reoperative cardiac surgery (74,75), cirrhosis (76), and obese or cachectic patients (<25 BMI >35) (77). The CORONARY trial also revealed a trend for improved outcomes in such patients in the highest EuroSCORE tertile (49). A recent large meta-analysis of RCTs concluded that off-pump can effectively reduce operative morbidity in high-risk patients (78). Consequently, European guidelines state that OPCAB is recommended in case of significant atherosclerotic aortic disease and it should be considered in high-risk patients but only by experienced off-pump surgeons (2).
MIDCAB or conventional CABG
Conventional CABG requires a sternotomy approach which is related to variable surgical trauma and subsequent wound complications. Minimally invasive approaches mainly aim to avoid these issues. MIDCAB was proposed in mid1990s (79-83) as a less invasive and attractive alternative for revascularization of isolated LAD stenosis (84). The LIMA-LAD anastomosis is performed on the beating heart through a left anterior small thoracotomy. Excellent results have been reported for MIDCAB in current literature. Immediate and 6-month angiographic patency rates of over 94% (82,85,86), a 24-month overall survival of 92.4%±0.2% and 24-month MACCE-free survival of 96.1%±1.7% at 24 months (87) are attributed to MIDCAB. Holzhey and colleagues reviewed their 13-year single-centre experience with MIDCAB in 1768 patients showing in-hospital mortality of 0.8% (despite EuroSCORE-predicted mortality of 3.8%), stroke rate of 0.4%, conversion to sternotomy rate of 1.7%, a 95.5% early patency rate and 3.3% early-reintervention rate. Remarkably 5- and 10-year survival rates were 88.3% and 76.6% respectively whereas 85.3% and 70.9% were the corresponding freedom from MACCE and angina (88). However, there was no superiority of MIDCAB regarding operative mortality, early myocardial infarction or stroke, late survival and need for repeat revascularization at a mean follow-up of 6.2 years compared to full sternotomy approaches (89,90). Furthermore, MIDCAB is a technically demanding procedure which is appropriate for selective patients and for selective, experienced surgeons. It is estimated that 100-150 MIDCAB cases are necessary to achieve acceptable complication and conversion to sternotomy rates (91). European guidelines report that MIDCAB should only be considered in isolated LAD lesions or as a part of hybrid procedures, provided that expertise exists (2). Subsequently, MIDCAB is currently adopted by a minority of dedicated centres and it is performed by few expert surgeons (92).
Evolution of minimally invasive techniques continues. Therefore, robotic CABG, as well as totally endoscopic coronary bypass grafting (TECAB) have been performed by experienced surgical teams showing excellent results, similar to those with conventional CABG (93-99). However, technical demands are increased and intense training is required thus rendering these approaches even more difficult to reproduce (92).
HCR or conventional CABG?
Parallel to minimally invasive procedures, the concept of HCR, firstly described by Angelini et al. in 1996 (100) has also gained popularity combining advantages of both percutaneous and surgical approaches. The rationale of HCR consists of a surgical LIMA-LAD anastomosis accompanied with PCI to the other abnormal non-LAD territories (101). According to Harskamp et al. (102), the LIMA-LAD anastomosis is superior to coronary stenting, whereas DES coronary stents are not inferior to venous grafts to non-LAD territories (102). Clinical outcomes after HCR are comparable to outcomes after conventional CABG, but only as far as experienced minimally invasive revascularization centres are concerned (103). However, no study has managed to show superiority of HCR over conventional CABG so far (104-107). Even in these experienced minimally invasive revascularization centres, although similar results in terms of survival and freedom from MACCE rates are reported, HCR is significantly inferior to conventional CABG regarding the need for repeat revascularization, mainly due to in stent restenosis or stent thrombosis (102,108). The randomized POL-MIDES study of 200 patients compared two groups of patients with multivessel CAD randomly assigned to conventional CABG or HCR. No significant difference in all-cause mortality, myocardial infarction, stroke, repeat coronary revascularization and MACCE at 1 year and at 5 years was detected (107,109). Similarly, the most recent, randomized, pilot study, the MERSING trial (101), comparing 40 hybrid patients with three-vessel CAD with 20 conventional CABG patients revealed no significant differences in terms of late safety and efficacy. The 2-year major cardiovascular event rate including death, myocardial infarction, stroke or repeat revascularization was 19.3% in the hybrid group versus 5.9% in the conventional group (P=0.2) (101). According to 2018 ESC/EACTS guidelines on myocardial revascularization, HCR has a IIb recommendation for specific patient subsets at experienced centres (2). Although relatively healthy patients with CAD may benefit from conventional CABG, avoiding in-stent restenosis risk, those who are unfit for conventional CABG due to advanced age, frailty, obesity, lack of conduits, poor non-LAD target vessels and porcelain aorta are candidates for HCR (103,108).
Saphenous vein grafts
Apart from sternotomy approach and cardiopulmonary bypass use, SVG harvesting is typical of conventional CABG. In practice, SVG remains the second most commonly used conduit in CABG after LIMA. It is a graft that can be easily harvested; it is available in abundance; it is versatile; it is minimally affected by spasm and there are plenty of studies exploring its long-term results. However, its late graft patency is questionable. There is plenty of data in the literature addressing the higher failure rates of SVGs. Various strategies like venous external stents, no-touch SVG harvesting techniques and post-harvesting vein storage in various preservation solutions along with optimal post-CABG medical treatment have been studied to indicate their impact on vein graft failure (92).
Many studies report the significance of optimal medical treatment for reducing post-CABG mortality. Aspirin administration within 48 hours postoperatively reduces postoperative mortality, myocardial infarction, stroke, renal failure and bowel infarction (110). Graft atherosclerosis rate and subsequent need for repeat revascularization is reduced by aggressive use of lipid-lowering agents to achieve a low-density lipoprotein cholesterol less than 100 mg/dL (111,112). As far as the technical part is concerned, an SVG can be harvested either openly or endoscopically (2). The latter approach leads to significantly less leg wound complications (113-116) which reduces morbidity of conventional CABG. Although there is some evidence of detrimental effect of endoscopic vein harvesting on graft patency (117), randomized and non-randomized trials do not demonstrate inferior clinical outcomes with endoscopic vein harvest (113,114,118,119). The most recent randomized REGROUP trial demonstrated no significant difference between the two approaches (120). However, if the endoscopic approach is chosen, it should only be performed by experienced, high-volume personnel (121-123). Furthermore, harvesting the vein with a pedicle of surrounding tissue via the “no-touch” technique has gained popularity. According to a randomized study of 104 patients, the no-touch technique is related to significantly better angiographic patency rates at 18 months (95%) compared to conventional harvesting technique (89%). No-touch vein graft patency rate was also remarkably superior at 8.5 years (90% vs. 76%, respectively). The most important technical factors affecting graft patency were the harvesting technique as well as the vein quality (124). Superior patency rates of no-touch harvested SVGs have been reported in multiple randomized trials (125-128), with a patency rate >80% at 16 years post-CABG (128). Hence, it is critical to take care of the saphenous vein during harvesting similar to the caution exercised during LIMA harvesting. Table 1 provides with a thorough registration of advantages and disadvantages of each one of the aforementioned approaches.
Table 1
| Pros | Cons | |
|---|---|---|
| Conventional CABG | Shorter learning curve | Inferior patency rates of SVGs |
| Appropriate for every patient | Cardioplegic arrest requirement | |
| The most long-standing approach | SIRS related to CPB | |
| Motionless and bloodless field | More surgical trauma and wound complications | |
| Low DSWI rates | Poorer cosmetic result | |
| Independent of native coronary vessel stenosis | ||
| SVGs minimally affected by spasm | ||
| More easily reproducible and teachable | ||
| Less need for repeat revascularization | ||
| BIMA grafting | Superior patency rates of RIMA | Appropriate only for selective patients |
| More technically demanding | ||
| Higher DSWI rates | ||
| TAR | Superior patency rates of RIMA/RA | RA choice only in case of native coronary vessel stenosis >90% |
| RA seriously affected by spasm | ||
| Off-pump CABG | Improved results in-high risk patients | Longer learning curve |
| No cardioplegia required | More technically demanding | |
| No CPB-related complications | Mobile and bloody field | |
| Higher incomplete revascularization rates | ||
| Possible conversion to conventional CABG | ||
| Increased mortality and morbidity after emergent conversion | ||
| Less easily reproducible and teachable | ||
| Only by experienced off-pump surgeons | ||
| Only in high volume centres | ||
| MIDCAB | Optimal cosmetic result | Longer learning curve |
| Less surgical trauma and wound complications | Appropriate only for selective patients | |
| More technically demanding | ||
| Less easily reproducible and teachable | ||
| Only by expert surgeons | ||
| Only in high volume centres | ||
| Possible conversion to conventional CABG | ||
| Increased mortality and morbidity after emergent conversion | ||
| HCR | Appropriate for unfit for conventional CABG patients | Appropriate only for selective patients |
| Appropriate for poor non-LAD target vessels | Stent restenosis risk | |
| Appropriate in case of porcelain aorta | More repeat revascularization rates | |
| Less surgical trauma and wound complications | Less easily reproducible and teachable | |
| Possible conversion to conventional CABG | ||
| Increased mortality and morbidity after emergent conversion |
BIMA, bilateral internal mammary artery; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; DSWI, deep sternal wound infection; HCR, hybrid coronary revascularization; LAD, left anterior descending; MIDCAB, minimal invasive coronary artery bypass; RA, radial artery; RIMA, right internal mammary artery; SIRS, systemic inflammatory response syndrome; SVGs, saphenous vein grafts; TAR, total arterial revascularization.
Conclusions
CABG surgery has remarkably evolved since its introduction by Dr. Favaloro in 1969. Conventional CABG is the cardiac surgeon’s “bread and butter”. Nowadays, conventional CABG faces challenge from several alternative approaches. BIMA grafting, TAR, OPCAB, MIDCAB or HCR are potential candidates to replace it. However, conventional CABG appears non-inferior to any of the aforementioned approaches (1,2,5,26,47-49,101). Moreover, conventional CABG is for every patient, as well as for every surgeon.
Contrary to its name, conventional CABG is contemporary. It is crucial to improve the outcomes of conventional approach further with optimal post-CABG medical treatment and adoption of modern approaches like no-touch or endoscopic SVG harvesting. Conventional CABG is easily reproducible and continues to evolve. It is not the shadow but the soul of innovations. Conventional CABG sheds light on the path of evolution. Thank you Dr. Favaloro…!
Acknowledgments
I would like to wholeheartedly thank Mr Shahzad Raja for kindly trusting me for the production of this project.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Shahzad G. Raja) for the series “Coronary Artery Bypass Grafting” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj.2020.03.15/coif). The series “Coronary Artery Bypass Grafting” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cohn L, Adams D. Cardiac surgery in the adult. 5th ed. McGraw-Hill Education. 2017;20:471-517.
- Sousa-Uva M, Neumann FJ, Ahlsson AESC Scientific Document Group, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2019;55:4-90. [Crossref] [PubMed]
- Favaloro RG, Effler DB, Groves LK, et al. Direct myocardial revascularization with saphenous vein autograft. Clinical experience in 100 cases. Dis Chest 1969;56:279-83. [Crossref] [PubMed]
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961-72. [Crossref] [PubMed]
- Shroyer AL, Grover FL, Hattler BVeterans Affairs Randomized On/Off Bypass (ROOBY) Study Group, et al. On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med 2009;361:1827-37. [Crossref] [PubMed]
- Carrel A. On the experimental surgery of the thoracic aorta and heart. Ann Surg 1910;52:83-95. [Crossref] [PubMed]
- Vineberg AM. Restoration of coronary circulation by anastomosis. Can Med Assoc J 1946;55:117-9. [PubMed]
- Vineberg AM, Jewett BL. Development of an anastomosis between the coronary vessels and a transplanted internal mammary artery. Can Med Assoc J 1947;56:609-14. [PubMed]
- Vineberg AM, Niloff PH. The value of surgical treatment of coronary artery occlusion by implantation of the internal mammary artery into the ventricular myocardium; an experimental study. Surg Gynecol Obstet 1950;91:551-61. [PubMed]
- Mueller RL, Rosengart TK, Isom OW. The history of surgery for ischemic heart disease. Ann Thorac Surg 1997;63:869-78. [Crossref] [PubMed]
- Mehta NJ, Khan IA. Cardiology's 10 Greatest Discoveries of the 20th Century. Tex Heart Inst J 2002;29:164-71. [PubMed]
- Favaloro RG, Effler DB, Cheanvechai C, et al. Acute coronary insufficiency (impending myocardial infarction and myocardial infarction): surgical treatment by the saphenous vein graft technique. Am J Cardiol 1971;28:598-607. [Crossref] [PubMed]
- Favaloro RG. Saphenous vein graft in the surgical treatment of coronary artery disease: operative technique. J Thorac Cardiovasc Surg 1969;58:178-85. [Crossref] [PubMed]
- Post PN, Kuijpers M, Ebels T, et al. The relation between volume and outcome of coronary interventions: a systematic review and metaanalysis. Eur Heart J 2010;31:1985-92. [Crossref] [PubMed]
- Kim LK, Looser P, Feldman DN. Peri- and postoperative care after coronary artery bypass grafting in low versus high volume centers. J Thorac Cardiovasc Surg 2016;152:1205. [Crossref] [PubMed]
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med 2003;349:2117-27. [Crossref] [PubMed]
- Lapar DJ, Mery CM, Kozower BD, et al. The effect of surgeon volume on mortality for off-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg 2012;143:854-63. [Crossref] [PubMed]
- Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2014;64:1929-49. [Crossref] [PubMed]
- Taggart DP, D’Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet 2001;358:870-5. [Crossref] [PubMed]
- Aldea GS, Bakaeen FG, Pal J, et al. The Society of Thoracic Surgeons Clinical Practice guidelines on arterial conduits for coronary artery bypass grafting. Ann Thorac Surg 2016;101:801-9. [Crossref] [PubMed]
- Itagaki S, Cavallaro P, Adams DH, et al. Bilateral internal mammary artery grafts, mortality and morbidity: an analysis of 1 526 360 coronary bypass operations. Heart 2013;99:849. [Crossref] [PubMed]
- Weiss AJ, Zhao S, Tian DH, et al. A meta-analysis comparing bilateral internal mammary artery with left internal mammary artery for coronary artery bypass grafting. Ann Cardiothorac Surg 2013;2:390-400. [PubMed]
- Buttar SN, Yan TD, Taggart DP, et al. Long-term and short-term outcomes of using bilateral internal mammary artery grafting versus left internal mammary artery grafting: a meta-analysis. Heart 2017;103:1419-26. [Crossref] [PubMed]
- Lytle BW, Blackstone EH, Loop FD, et al. Two internal thoracic artery grafts are better than one. J Thorac Cardiovasc Surg 1999;117:855-72. [Crossref] [PubMed]
- Tatoulis J, Buxton BF, Fuller JA. The right internal thoracic artery: the forgotten conduit — 5,766 patients and 991 angiograms. Ann Thorac Surg 2011;92:9-15. [Crossref] [PubMed]
- Taggart DP, Benedetto U, Gerry SArterial Revascularization Trial Investigators, et al. Bilateral versus Single Internal-Thoracic-Artery Grafts at 10 Years. N Engl J Med 2019;380:437-46. [Crossref] [PubMed]
- Taggart DP, Altman DG, Gray AM, et al. Randomized trial of bilateral versus single internal-thoracic-artery grafts. N Engl J Med 2016;375:2540-9. [Crossref] [PubMed]
- Loop FD, Lytle BW, Cosgrove DM, et al. J. Maxwell Chamberlain memorial paper. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg 1990;49:179-186; discussion 186-187. [Crossref] [PubMed]
- Gansera B, Schmidtler F, Gillrath G, et al. Does bilateral ITA grafting increase perioperative complications? Outcome of 4462 patients with bilateral versus 4204 patients with single ITA bypass. Eur J Cardiothorac Surg 2006;30:318-23. [Crossref] [PubMed]
- Toumpoulis IK, Theakos N, Dunning J. Does bilateral internal thoracic artery harvest increase the risk of mediastinitis? Interact Cardiovasc Thorac Surg 2007;6:787-91. [Crossref] [PubMed]
- Milano CA, Kesler K, Archibald N, et al. Mediastinitis after coronary artery bypass graft surgery. Risk factors and long-term survival. Circulation 1995;92:2245-51. [Crossref] [PubMed]
- Parisian Mediastinitis Study Group. Risk factors for deep sternal wound infection after sternotomy: a prospective, multicenter study. J Thorac Cardiovasc Surg 1996;111:1200-7. [Crossref] [PubMed]
- Olsen MA, Lock-Buckley P, Hopkins D, et al. The risk factors for deep and superficial chest surgical-site infections after coronary artery bypass graft surgery are different. J Thorac Cardiovasc Surg 2002;124:136-45. [Crossref] [PubMed]
- Kieser TM, Lewin AM, Graham MMAPPROACH Investigators, et al. Outcomes associated with bilateral internal thoracic artery grafting: the importance of age. Ann Thorac Surg 2011;92:1269-75; discussion 1275-1276. [Crossref] [PubMed]
- Yi G, Shine B, Rehman SM, et al. Effect of bilateral internal mammary artery grafts on long-term survival: A meta-analysis approach. Circulation 2014;130:539-45. [Crossref] [PubMed]
- Benedetto U, Amrani M, Raja SGHarefield Cardiac Outcomes Research Group. Guidance for the use of bilateral internal thoracic arteries according to survival benefit across age groups. J Thorac Cardiovasc Surg 2014;148:2706-11. [Crossref] [PubMed]
- ElBardissi AW, Aranki SF, Sheng S, et al. Trends in isolated coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons adult cardiac surgery database. J Thorac Cardiovasc Surg 2012;143:273-81. [Crossref] [PubMed]
- Gaudino M, Taggart D, Suma H, et al. The choice of conduits in coronary artery bypass surgery. J Am Coll Cardiol 2015;66:1729-37. [Crossref] [PubMed]
- Buxton BF, Shi WY, Tatoulis J, et al. Total arterial revascularization with internal thoracic and radial artery grafts in triple-vessel coronary artery disease is associated with improved survival. J Thorac Cardiovasc Surg 2014;148:1238-1243; discussion 1243-1244. [Crossref] [PubMed]
- Gaudino M, Benedetto U, Fremes S, et al. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N Engl J Med 2018;378:2069-77. [Crossref] [PubMed]
- Hayward PA, Gordon IR, Hare DL, et al. Comparable patencies of the radial artery and right internal thoracic artery or saphenous vein beyond 5 years: results from the Radial Artery Patency and Clinical Outcomes trial. J Thorac Cardiovasc Surg 2010;139:60-5; discussion 65-67. [Crossref] [PubMed]
- Goldman S, Sethi GK, Holman W, et al. Radial artery grafts vs saphenous vein grafts in coronary artery bypass surgery: a randomized trial. JAMA 2011;305:167-74. [Crossref] [PubMed]
- Desai ND, Cohen EA, Naylor CD, et al. A randomized comparison of radial-artery and saphenous-vein coronary bypass grafts. N Engl J Med 2004;351:2302-9. [Crossref] [PubMed]
- Maniar HS, Barner HB, Bailey MS, et al. Radial artery patency: are aortocoronary conduits superior to composite grafting? Ann Thorac Surg 2003;76:1498-1503; discussion 1503-1504. [Crossref] [PubMed]
- Hadinata IE, Hayward PA, Hare DL, et al. Choice of conduit for the right coronary system: 8-year analysis of Radial Artery Patency and Clinical Outcomes trial. Ann Thorac Surg 2009;88:1404-9. [Crossref] [PubMed]
- Tatoulis J, Buxton BF, Fuller JA, et al. Long-term patency of 1108 radial arterial-coronary angiograms over 10 years. Ann Thorac Surg 2009;88:23-9; discussion 29-30. [Crossref] [PubMed]
- Diegeler A, Borgermann J, Kappert UGOPCABE Study Group, et al. Off-pump versus on-pump coronary-artery bypass grafting in elderly patients. N Engl J Med 2013;368:1189-98. [Crossref] [PubMed]
- Lamy A, Devereaux PJ, Prabhakaran D, et al. CORONARY Investigators. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012;366:1489-97. [Crossref] [PubMed]
- Lamy A, Devereaux PJ, Prabhakaran D, et al. CORONARY Investigators. Effects of off-pump and on-pump coronary artery bypass grafting at 1 year. N Engl J Med 2013;368:1179-88. [Crossref] [PubMed]
- Hattler B, Messenger JC, Shroyer ALVeterans Affairs Randomized On/Off Bypass (ROOBY) Study Group, et al. Off-pump coronary artery bypass surgery is associated with worse arterial and saphenous vein graft patency and less effective revascularization: Results from the Veterans Affairs Randomized On/Off Bypass (ROOBY) trial. Circulation 2012;125:2827-35. [Crossref] [PubMed]
- Houlind K, Kjeldsen BJ, Madsen SNDOORS Group, et al. On-pump versus off-pump coronary artery bypass surgery in elderly patients: Results from the Danish on-pump versus off-pump randomization study. Circulation 2012;125:2431-9. [Crossref] [PubMed]
- Hannan EL, Wu C, Smith CR, et al. Off-pump versus on-pump coronary artery bypass graft surgery: differences in short-term outcomes and in long-term mortality and need for subsequent revascularization. Circulation 2007;116:1145-52. [Crossref] [PubMed]
- Jones EL, Weintraub WS. The importance of completeness of revascularization during long-term follow-up after coronary artery operations. J Thorac Cardiovasc Surg 1996;112:227-37. [Crossref] [PubMed]
- Synnergren MJ, Ekroth R, Oden A, et al. Incomplete revascularization reduces survival benefit of coronary artery bypass grafting: role of off-pump surgery. J Thorac Cardiovasc Surg 2008;136:29-36. [Crossref] [PubMed]
- Benedetto U, Altman DG, Gerry SArterial Revascularization Trial Investigators, et al. Off-pump versus On-pump coronary artery bypass grafting. Insights from the Arterial Revascularization Trial. J Thorac Cardiovasc Surg 2018;155:1545-53.e7. [Crossref] [PubMed]
- Lamy A, Devereaux PJ, Prabhakaran D, et al. CORONARY Investigators. Five-Year Outcomes after Off-Pump or On-Pump Coronary-Artery Bypass Grafting. N Engl J Med 2016;375:2359-68. [Crossref] [PubMed]
- Takagi H, Umemoto TAll-Literature Investigation of Cardiovascular Evidence (ALICE) Group. Worse long-term survival after off-pump than on-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg 2014;148:1820-9. [Crossref] [PubMed]
- Raja SG, Garg S, Soni MK. On-pump and off-pump coronary artery bypass grafting for patients needing at least two grafts: comparative outcomes at 20 years. Eur J Cardiothorac Surg 2020;57:512-9. [PubMed]
- Urso S, Sadaba JR, Pettinari M. Impact of off-pump to on-pump conversion rate on post-operative results in patients undergoing off-pump coronary artery bypass. Interact Cardiovasc Thorac Surg 2012;14:188-93. [Crossref] [PubMed]
- Edgerton JR, Dewey TM, Magee MJ, et al. Conversion in off-pump coronary artery bypass grafting: an analysis of predictors and outcomes. Ann Thorac Surg 2003;76:1138-42. [Crossref] [PubMed]
- Mathur AN, Pather R, Widjanarko J, et al. Off-pump coronary artery bypass: the Sudbury experience. Can J Cardiol 2003;19:1261-9. [PubMed]
- Soltoski P, Salerno T, Levinsky L, et al. Conversion to cardiopulmonary bypass in off-pump coronary artery bypass grafting: its effect on outcome. J Card Surg 1998;13:328-34. [Crossref] [PubMed]
- Iacò AL, Contini M, Teodori G, et al. Off or on bypass: what is the safety threshold? Ann Thorac Surg 1999;68:1486-9. [Crossref] [PubMed]
- Mujanovic E, Kabil E, Hadziselimovic M, et al. Conversions in off-pump coronary surgery. Heart Surg Forum 2003;6:135-7. [Crossref] [PubMed]
- Puskas JD, Edwards FH, Pappas PA, et al. Off-pump techniques benefit men and women and narrow the disparity in mortality after coronary bypass grafting. Ann Thorac Surg 2007;84:1447-54. [Crossref] [PubMed]
- Youn YN, Chang BC, Hong YS, et al. Early and mid-term impacts of cardiopulmonary bypass on coronary artery bypass grafting in patients with poor left ventricular dysfunction: a propensity score analysis. Circ J 2007;71:1387-94. [Crossref] [PubMed]
- Darwazah AK, Abu Sham’a RA, Hussein E, et al. Myocardial revascularizationin patients with low ejection fraction < or =35%: effect of pump technique on early morbidity and mortality. J Card Surg 2006;21:22-7. [Crossref] [PubMed]
- Fattouch K, Guccione F, Dioguardi P, et al. Off-pump versus on-pump myocardial revascularization in patients with ST-segment elevation myocardial infarction: a randomized trial. J Thorac Cardiovasc Surg 2009;137:650-6. [Crossref] [PubMed]
- Halkos ME, Puskas JD, Lattouf OM, et al. Impact of preoperative neurologic events on outcomes after coronary artery bypass grafting. Ann Thorac Surg 2008;86:504-10. [Crossref] [PubMed]
- Mishra YK, Collison SP, Malhotra R, et al. Ten-year experience with single-vessel and multivessel reoperative off-pump coronary artery bypass grafting. J Thorac Cardiovasc Surg 2008;135:527-32. [Crossref] [PubMed]
- Dewey TM, Herbert MA, Prince SL, et al. Does coronary artery bypass graft surgery improve survival among patients with end-stage renal disease? Ann Thorac Surg 2006;81:591-8. [Crossref] [PubMed]
- García Fuster R, Paredes F, García Peláez A, et al. Impact of increasing degrees of renal impairment on outcomes of coronary artery bypass grafting: the off-pump advantage. Eur J Cardiothorac Surg 2013;44:732-42. [Crossref] [PubMed]
- Chawla LS, Zhao Y, Lough FC, et al. Off-pump vs. on-pump coronary artery bypass grafting outcomes stratified by preoperative renal function. J Am Soc Nephrol 2012;23:1389-97. [Crossref] [PubMed]
- Sepehripour AH, Harling L, Ashrafian H, et al. Does off-pump coronary revascularization confer superior organ protection in re-operative coronary artery surgery? A meta-analysis of observational studies. J Cardiothorac Surg 2014;9:115. [Crossref] [PubMed]
- Dohi M, Miyata H, Doi K, et al. The off-pump technique in redo coronary artery bypass grafting reduces mortality and major morbidities: propensity score analysis of data from the Japan Cardiovascular Surgery Database. Eur J Cardiothorac Surg 2015;47:299-307. [Crossref] [PubMed]
- Gopaldas RR, Chu D, Cornwell LD, et al. Cirrhosis as a moderator of outcomes in coronary artery bypass grafting and off-pump coronary artery bypass operations: a 12-year population-based study. Ann Thorac Surg 2013;96:1310-5. [Crossref] [PubMed]
- Keeling WB, Kilgo PD, Puskas JD, et al. Off-pump coronary artery bypass grafting attenuates morbidity and mortality for patients with low and high body mass index. J Thorac Cardiovasc Surg 2013;146:1442-8. [Crossref] [PubMed]
- Kowalewski M, Pawliszak W, Malvindi PG, et al. Off-pump coronary artery bypass grafting improves short-term outcomes in high-risk patients compared with on-pump coronary artery bypass grafting: Meta analysis. J Thorac Cardiovasc Surg 2016;151:60-77.e1. [Crossref] [PubMed]
- Calafiore AM, Giammarco GD, Teodori G, et al. Left anterior descending coronary artery grafting via left anterior small thoracotomy without cardiopulmonary bypass. Ann Thorac Surg 1996;61:1658-1663;discussion 1664-1655.
- Cremer J, Struber M, Wittwer T, et al. Off-bypass coronary bypass grafting via minithoracotomy using mechanical epicardial stabilization. Ann Thorac Surg 1997;63:S79-83. [Crossref] [PubMed]
- Subramanian VA, McCabe JC, Geller CM. Minimally invasive direct coronary artery bypass grafting: two-year clinical experience. Ann Thorac Surg 1997;64:1648-1653; discussion 1654-1655. [Crossref] [PubMed]
- Diegeler A, Matin M, Kayser S, et al. Angiographic results after minimally invasive coronary bypass grafting using the minimally invasive direct coronary bypass grafting (midcab) approach. Eur J Cardiothorac Surg 1999;15:680-4. [Crossref] [PubMed]
- Bucerius J, Metz S, Walther T, et al. Endoscopic internal thoracic artery dissection leads to significant reduction of pain after minimally invasive direct coronary artery bypass graft surgery. Ann Thorac Surg 2002;73:1180-4. [Crossref] [PubMed]
- Head SJ, Borgermann J, Osnabrugge RL, et al. Coronary artery bypass grafting: Part 2: optimizing out- comes and future prospects. Eur Heart J 2013;34:2873-86. [Crossref] [PubMed]
- Mack MJ, Magovern JA, Acuff TA, et al. Results of graft patency by immediate angiography in minimally invasive coronary artery surgery. Ann Thorac Surg 1999;68:383-389; discussion 389-390. [Crossref] [PubMed]
- Kettering K, Dapunt O, Baer FM. Minimally invasive direct coronary artery bypass grafting: a systematic review. J Cardiovasc Surg (Torino) 2004;45:255-64. [PubMed]
- Reser D, Hemelrijck M, Pavicevic J, et al. Mid-term outcomes of minimally invasive direct coronary artery bypass grafting. Thorac Cardiovasc Surg 2015;63:313-8. [PubMed]
- Holzhey DM, Cornely JP, Rastan AJ, et al. Review of a 13-year single center experience with minimally invasive direct coronary artery bypass as the primary surgical treatment of coronary artery disease. Heart Surg Forum 2012;15:E61-8. [Crossref] [PubMed]
- Raja SG, Benedetto U, Alkizwini E, et al. Propensity score adjusted comparison of midcab versus full sternotomy left anterior descending artery revascularization. Innovations (Phila) 2015;10:174-8. [Crossref] [PubMed]
- Birla R, Patel P, Aresu G, et al. Minimally invasive direct coronary artery bypass versus off-pump coronary surgery through sternotomy. Ann R Coll Surg Engl 2013;95:481-5. [Crossref] [PubMed]
- Holzhey DM, Jacobs S, Walther T, et al. Cumulative sum failure analysis for eight surgeons performing minimally invasive direct coronary artery bypass. J Thorac Cardiovasc Surg 2007;134:663-9. [Crossref] [PubMed]
- Caliskan E, Emmert MY, Falk V. What will surgical coronary revascularization look like in 25 years? Curr Opin Cardiol 2019;34:637-44. [Crossref] [PubMed]
- Mack MJ, Brown DL, Sankaran A. Minimally invasive coronary bypass for protected left main coronary stenosis angioplasty. Ann Thorac Surg 1997;64:545-6. [Crossref] [PubMed]
- Friedrich GJ, Bonatti J. Hybrid coronary artery revascularization—review and update 2007. Heart Surg Forum 2007;10:E292-6. [Crossref] [PubMed]
- Katz MR, Van Praet F, de Canniere D, et al. Integrated coronary revascularization: percutaneous coronary intervention plus robotic totally endoscopic coronary artery bypass. Circulation 2006;114:I473-6. [Crossref] [PubMed]
- Halkos ME, Liberman HA, Devireddy C, et al. Early clinical and angiographic outcomes after robotic-assisted coronary artery bypass surgery. J Thorac Cardiovasc Surg 2014;147:179-85. [Crossref] [PubMed]
- Leonard JR, Rahouma M, Abouarab AA, et al. Totally endoscopic coronary artery bypass surgery: a meta-analysis of the current evidence. Int J Cardiol 2018;261:42-6. [Crossref] [PubMed]
- Göbölös L, Ramahi J, Obeso A, et al. Robotic totally endoscopic coronary artery bypass grafting: systematic review of clinical outcomes from the past two decades. Innovations 2019;14:5-16. [Crossref] [PubMed]
- Kitahara H, Nisivaco S, Balkhy HH. Graft patency after robotically assisted coronary artery bypass surgery. Innovations 2019;14:117-23. [Crossref] [PubMed]
- Angelini GD, Wilde P, Salerno TA, et al. Integrated left small thoracotomy and angioplasty for multivessel coronary artery revascularisation. Lancet 1996;347:757-8. [Crossref] [PubMed]
- Esteves V, Oliveira MAP, Feitosa FS, et al. Late clinical outcomes of myocardial hybrid revascularization versus coronary artery bypass grafting for complex triple-vessel disease: Long‐term follow-up of the randomized MERGING clinical trial. Catheter Cardiovasc Interv 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Harskamp RE, Bagai A, Halkos ME, et al. Clinical outcomes after hybrid coronary revascularization versus coronary artery bypass surgery: a meta-analysis of 1190 patients. Am Heart J 2014;167:585-92. [Crossref] [PubMed]
- Harskamp RE, Puskas JD, Tijssen JG, et al. Comparison of hybrid coronary revascularization versus coronary artery bypass grafting in patients ≥65 years with multivessel coronary artery disease. Am J Cardiol 2014;114:224-9. [Crossref] [PubMed]
- Reynolds AC, King N. Hybrid coronary revascularization versus conventional coronary artery bypass grafting: systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e11941. [Crossref] [PubMed]
- Sardar P, Kundu A, Bischoff M, et al. Hybrid coronary revascularization versus coronary artery bypass grafting in patients with multivessel coronary artery disease: a meta-analysis. Catheter Cardiovasc Interv 2018;91:203-12. [Crossref] [PubMed]
- Modrau IS, Holm NR, Maeng M, et al. One-year clinical and angiographic results of hybrid coronary revascularization. J Thorac Cardiovasc Surg 2015;150:1181-6. [Crossref] [PubMed]
- Gąsior M, Zembala MO, Tajstra MPOL-MIDES (HYBRID) Investigators, et al. Hybrid revascularization for multivessel coronary artery disease. JACC Cardiovasc Interv 2014;7:1277-83. [Crossref] [PubMed]
- Halkos ME, Vassiliades TA, Douglas JS, et al. Hybrid coronary revascularization versus off-pump coronary artery bypass grafting for the treatment of multivessel coronary artery disease. Ann Thorac Surg 2011;92:1695-701. [Crossref] [PubMed]
- Tajstra M, Hrapkowicz T, Hawranek MPOL-MIDES Study Investigators, et al. Hybrid coronary revascularization in selected patients with multivessel disease: 5-year clinical outcomes of the prospective randomized pilot study. JACC Cardiovasc Interv 2018;11:847-52. [Crossref] [PubMed]
- Mangano DTMulticenter Study of Perioperative Ischemia Research Group. Aspirin and mortality from coronary bypass surgery. N Engl J Med 2002;1309-17. [Crossref] [PubMed]
- Post Coronary Artery Bypass Graft Trial Investigators. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med 1997;336:153-62. [Crossref] [PubMed]
- Hata M, Takayama T, Sezai A, et al. Efficacy of aggressive lipid controlling therapy for preventing saphenous vein graft disease. Ann Thorac Surg 2009;88:1440-4. [Crossref] [PubMed]
- Ouzounian M, Hassan A, Buth KJ, et al. Impact of endoscopic versus open saphenous vein harvest techniques on outcomes after coronary artery bypass grafting. Ann Thorac Surg 2010;89:403-8. [Crossref] [PubMed]
- Yun KL, Wu Y, Aharonian V, et al. Randomized trial of endoscopic versus open vein harvest for coronary artery bypass grafting: Six-month patency rates. J Thorac Cardiovasc Surg 2005;129:496-503. [Crossref] [PubMed]
- Chernyavskiy A, Volkov A, Lavrenyuk O, et al. Comparative results of endoscopic and open methods of vein harvesting for coronary artery bypass grafting: A prospective randomized parallel-group trial. J Cardiothorac Surg 2015;10:163. [Crossref] [PubMed]
- Krishnamoorthy B, Critchley WR, Glover AT, et al. A randomized study comparing three groups of vein harvesting methods for coronary artery bypass grafting: endoscopic harvest versus standard bridging and open techniques. Interact CardioVasc Thorac Surg 2012;15:224-8. [Crossref] [PubMed]
- Lopes RD, Hafley GE, Allen KB, et al. Endoscopic versus open veingraft harvesting in coronary-artery bypass surgery. N Engl J Med 2009;361:235-44. [Crossref] [PubMed]
- Deppe AC, Liakopoulos OJ, Choi YH, et al. Endoscopic vein harvesting for coronary artery bypass grafting: A systematic review with meta-analysis of 27789 patients. J Surg Res 2013;180:114-24. [Crossref] [PubMed]
- Williams JB, Peterson ED, Brennan JM, et al. Association between endoscopic vs open vein-graft harvesting and mortality, wound complications, and cardiovascular events in patients undergoing CABG surgery. JAMA 2012;308:475-84. [Crossref] [PubMed]
- Zenati MA, Bhatt DL, Bakaeen FGREGROUP Trial Investigators, et al. Randomized trial of endoscopic or open vein-graft harvesting for coronary-artery bypass. N Engl J Med 2019;380:132-41. [Crossref] [PubMed]
- Brown EN, Kon ZN, Tran R, et al. Strategies to reduce intraluminal clot formation in endoscopically harvested saphenous veins. J Thorac Cardiovasc Surg 2007;134:1259-65. [Crossref] [PubMed]
- Khaleel MS, Dorheim TA, Duryee MJ, et al. High-pressure distention of the saphenous vein during preparation results in increased markers of inflammation: A potential mechanism for graft failure. Ann Thorac Surg 2012;93:552-8. [Crossref] [PubMed]
- Rousou LJ, Taylor KB, Lu XG, et al. Saphenous vein conduits harvested by endoscopic technique exhibit structural and functional damage. Ann Thorac Surg 2009;87:62-70. [Crossref] [PubMed]
- Souza DS, Johansson B, Bojo L, et al. Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: results of a randomized longitudinal trial. J Thorac Cardiovasc Surg 2006;132:373-8. [Crossref] [PubMed]
- Johansson BL, Souza DS, Bodin L, et al. Slower progression of atherosclerosis in vein grafts harvested with ‘touch’ technique compared with conventional harvesting technique in coronary artery bypass grafting: An angiographic and intravascular ultrasound study. Eur J Cardiothorac Surg 2010;38:414-9. [Crossref] [PubMed]
- Souza DS, Dashwood MR, Tsui JC, et al. Improved patency in vein grafts harvested with surrounding tissue: Results of a randomized study using three harvesting techniques. Ann Thorac Surg 2002;73:1189-95. [Crossref] [PubMed]
- Dreifaldt M, Mannion JD, Bodin L, et al. The no-touch saphenous vein as the preferred second conduit for coronary artery bypass grafting. Ann Thorac Surg 2013;96:105-11. [Crossref] [PubMed]
- Samano N, Geijer H, Liden M, et al. The notouch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: A randomized trial. J Thorac Cardiovasc Surg 2015;150:880-8. [Crossref] [PubMed]
Cite this article as: Papakonstantinou NA. Conventional coronary artery bypass grafting. AME Med J 2020;5:38.

