
Narrative review of the surgical management of high-risk upper tract urothelial carcinoma
Introduction
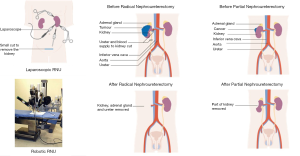
Upper tract urothelial carcinoma (UTUC) accounts for 5–10% of all urothelial malignancies, and it occurs between the level of the pyelocaliceal cavities in the kidneys and the distal ureters (1,2). UTUC typically presents with microscopic or gross hematuria and associated ipsilateral flank pain, which is confirmed using computed tomography (CT) or magnetic resonance imaging (MRI) urography imaging to detect a urothelial mass in the upper urinary collecting system (3). Further diagnostic work-up including ureteroscopy and biopsy may be necessary for pathological diagnosis or to determine tumor burden and location. The majority of UTUC (~75%) are classified as high-risk defined by any of the following features including (I) the presence of hydronephrosis, (II) tumor size greater than 2 cm, (III) high-grade cytology, (IV) high-grade pathological biopsy, (V) multifocal disease, (VI) previous radical cystectomy for bladder cancer, and (VII) variant histology (4). High-risk UTUC includes high-grade lesions on urine cytology or biopsy including carcinoma in situ (CIS), and locally invasive upper tract tumors (pathological stage pT1–T4) (5). The gold standard of care treatment for high-risk UTUC includes radical nephroureterectomy (RNU) with regional lymph node dissection (LND) and bladder cuff excision (6,7). Systemic platinum-based chemotherapy may also be utilized in a neoadjuvant or adjuvant fashion although clinical trials are ongoing. As shown in Figure 1, RNU with LND and bladder cuff excision may be performed via an open, laparoscopic, or robotic-assisted laparoscopic approach for high-risk UTUC. In this review, we summarize the various surgical procedures and treatment options in the management of high-risk UTUC including the advantage and disadvantages of each technique based on the established literature. We present the following article in accordance with the Narrative Review reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-2020-smgm-01/rc).
Methods
A comprehensive literature review was performed at the end of April 2020 within the PubMed database using the keyword search phrases “upper tract urothelial carcinoma” and “surgical management” with no date restriction. Initially, 527 articles were identified after the initial search, but 460 studies were excluded as they were not relevant to our topic of interest. Of the 67 studies included, 20 articles reported on laparoscopic RNU, 13 articles evaluated robotic-assisted RNU (RARNU), 10 articles examined various techniques of bladder cuff excision, 7 articles dealt with perioperative systemic therapy, 2 articles examined the utility of nephron-sparing surgery, 10 articles evaluated a single postoperative dose of intravesical chemotherapy, and 5 articles reported on regional lymphadenectomy. All studies included in our search were clinical research articles, systematic reviews, or meta-analysis. Our findings, including a review of the results, are summarized below.
Nephroureterectomy
The standard surgical management for oncological control of the primary tumor in high-risk UTUC is RNU (3). This treatment may be performed via an open, laparoscopic, or robotic-assisted laparoscopic approach. Open RNU is the traditional approach for excision of the primary malignancy in the surgical management of high-risk UTUC (3,8). It is typically performed through a flank or subcostal open incision with the patient lying in the supine or modified lateral position (3,8). The renal hilum including the renal arteries and vein is dissected and ligated and the lower portion of the ureter is clipped, dissected, and removed from the bladder cuff, which is sutured in two separate layers (8). The kidney with surrounding Gerota’s fascia and the entire ureteral length from the renal pelvis down to the bladder cuff is removed, and the adrenal gland is typically spared. The open RNU procedure is effective at removing distal ureteral tumors and allowing for accurate histological examination however this procedure can disrupt the structure and integrity of the bladder (8).
Laparoscopic RNU
Minimally-invasive laparoscopic RNU for UTUC has quickly replaced traditional open surgery due to its reduced morbidity, lower complication rates, shortened length of hospitalization, improved postoperative pain control, and quicker overall recovery (9-11).
A summary of the relevant literature for laparoscopic RNU in UTUC is shown in Table 1. An early study by Alothman et al. examining 24 patients who underwent laparoscopic RNU showed that laparoscopic surgery reduced operative time, blood loss, and postoperative hospital stay compared to traditional open surgery with similar rates of cancer-specific survival (CSS) during follow-up (75% vs. 73.3%, respectively) (12). Interestingly, patients who underwent a prior ureteroscopy with biopsy or had previous history of bladder cancer had an increased risk of developing bladder cancer recurrence after RNU (12). Future studies could investigate whether prior endoscopic manipulation could influence bladder cancer recurrence rates after laparoscopic RNU.
Table 1
| Author name | Year | Number of patients |
|---|---|---|
| Alothman et al. (12) | 2020 | 24 |
| Kim et al. (13) | 2019 | 615 |
| Kim et al. (14) | 2019 | 615 |
| Lee et al. (15) | 2019 | 137 |
| Nazzani et al. (16) | 2019 | 1,093 |
| Nouralizadeh et al. (17) | 2018 | N/A |
| Liu et al. (18) | 2018 | N/A |
| Zhang et al. (19) | 2016 | N/A |
| Hanske et al. (20) | 2015 | 599 |
| Sugihara et al. (21) | 2015 | 3,349 |
| Hanna et al. (22) | 2012 | 754 |
| Walton et al. (23) | 2010 | 70 |
| Kitamura et al. (24) | 2014 | 195 |
| Ariane et al. (25) | 2012 | 150 |
| Zou et al. (26) | 2014 | 101 |
| Blackmur et al. (27) | 2015 | 13 |
| Kido et al. (28) | 2018 | 48 |
| Liu et al. (29) | 2017 | 52 |
| Peyronnet et al. (30) | 2019 | 2,629 |
| Miyazaki et al. (31) | 2016 | 1,509 |
RUN, radical nephroureterectomy; N/A, not available.
Kim et al. subsequently showed an improved CSS and overall survival (OS) when comparing 615 laparoscopic RNU patients to 906 open RNU patients (80.4% vs. 76.4%, respectively, and 75.8% vs. 71.4%, respectively) (13). Kim et al. also observed higher 3-year CSS and OS for laparoscopic RNU patients (82.9% and 86.2%, respectively) compared to traditional open surgery (78.3% and 81.8%, respectively) in a cohort of 1,276 patients (14). The authors suggested that reduced operative time, blood loss, and hospitalization may explain the improved survival outcomes seen in their study.
Other studies by Lee et al. (15), Nazzani et al. (16), and a systematic review by Nouralizadeh et al. (17) showed similar reductions in length of hospital stay, blood loss, and postoperative complication rates when comparing laparoscopic to open RNU. The total cost of laparoscopic RNU, however, was higher than open RNU (16). These results were also confirmed in two separate meta-analysis and systematic reviews by Liu et al. and Zhang et al. except that no difference in postoperative complication rates between laparoscopic and open RNU were observed (18,19). Hanske et al. expanded on the benefits of laparoscopic RNU reporting that in a population of 599 patients, the overall incidence and risk of thromboembolic complications as well as operative re-intervention were reduced when compared to traditional open surgery (20).
Sugihara et al. reported that 3,349 patients who underwent laparoscopic RNU had longer anesthesia time and total operative costs (excluding operating room costs) with a lower 30-day postoperative mortality rate compared to 3,595 open RNU patients (21). Sugihara et al. observed no difference in overall postoperative complication rates between laparoscopic and open RNU although a subsequent study by Hanna et al. showed a reduced 30-day postoperative complication rate with the laparoscopic RNU procedure (22).
Despite the benefits of laparoscopic RNU, several studies suggest that open RNU may produce equivalent or superior outcomes comparatively. A study by Walton et al. showed that the CSS for laparoscopic and open RNU procedures were similar (75.2% vs. 75.4%, respectively) although the sample size in the laparoscopic group was relatively small (23). Similarly, Kitamura et al. showed no difference in CSS between laparoscopic and open RNU patients although recurrence-free survival (RFS) was improved in the laparoscopic group (33.8% vs. 41.2%, respectively) (24). A subsequent multi-center study by Ariane et al. found no difference in oncological outcomes including CSS and RFS between laparoscopic and open surgery although there was a gender disparity between groups (25). Zou et al. showed a similar 1-, 2- and 5-year CSS after open versus laparoscopic RNU for high-risk UTUC (92.1% vs. 95.2%, 87.1% vs. 90.5%, and 79.2% vs. 85.7%, respectively) with tumor stage, grade, and presence of lymphovascular invasion being the strongest predictors of cancer-specific death (26). Furthermore, patients with a history of bladder cancer and/or hydronephrosis were more likely to experience tumor recurrence after surgery (26).
A matched-paired analysis by Blackmur et al. to reduce potential confounders such as age, gender, and tumor stage further corroborated findings showing that laparoscopic and open RNU had comparable mean operative times, 5-year OS, RFS, CSS, and bladder cancer recurrence rate (27). Kido et al. found a similar result after propensity score matching for baseline clinical characteristics, which support observations of potential confounders causing laparoscopic RNU to appear superior to open RNU procedures (28). In a subgroup of patients with locally advanced, high-risk UTUC (pT3/T4, N+) who underwent laparoscopic versus open RNU, Liu et al. reported no difference in 5-year RFS (47% vs. 59%, respectively), CSS (63% vs. 70%, respectively), and OS (61% vs. 55%, respectively) (29). A systematic review by Peyronnet et al. found that oncological outcomes were actually worse in laparoscopic compared to open RNU for patients with locally advanced, high-risk UTUC (30) while Miyazaki et al. found that CSS was similar in patients with localized, muscle-invasive (pT2) UTUC who underwent laparoscopic versus open surgery (31).
RARNU
Despite the postoperative improvements for patients, the laparoscopic RNU approach can prove to be difficult to master with a steep learning curve given its limited visualization, tactile feedback, and limited work space within the pelvis (32). In 2006, minimally-invasive robotic-assisted laparoscopic surgery further evolved and transitioned to RNU surgery, alleviating a lot of the technical challenges associated with laparoscopic surgery with enhanced 3D visualization and magnification, improved manual dexterity with 360-degree wrist articulation, and improved access to the pelvis with a reduction in patient repositioning for bladder cuff excision (33-35). Furthermore, RARNU may prevent tumor spillage due to enhancing manual dexterity and allow for more accurate and appropriate LND in the retroperitoneum and pelvis (33-35). Compared to traditional laparoscopic RNU, RARNU surgery allows the surgeon a smooth and wide range of motion with improved visualization and magnification (36). The robotic-assisted technique also allows for better visualization of the renal and pelvic vasculature as well as surrounding tissues, which can decrease surgical complications especially in locally advanced cases (37-40).
A summary of the relevant literature for RARNU in UTUC is shown in Table 2. Aboumohamed et al. found that RARNU with bladder cuff excision improved 2- and 5-year RFS (65.3% and 57.1%), CSS (92.9% and 69.5%), and OS (86.9% and 62.6%) compared with historical data (37). Furthermore, this study reported that RNU in elderly patients with preoperative hydronephrosis, nodal disease, concomitant CIS, lymphovascular invasion, or impaired preoperative renal function was associated with lower RFS (37). Several smaller studies have reported the feasibility of RARNU for high-risk UTUC with acceptable recurrence risk (38-40).
Table 2
| Author name | Year | Number of patients |
|---|---|---|
| Aboumohamed et al. (37) | 2015 | 65 |
| Eandi et al. (38) | 2010 | 11 |
| Pugh et al. (39) | 2013 | 43 |
| Yang et al. (40) | 2014 | 26 |
| De Groote et al. (41) | 2020 | 78 |
| Lim et al. (42) | 2013 | 32 |
| Mullen et al. (43) | 2017 | N/A |
| Pathak et al. (44) | 2018 | 204 |
| Ribal et al. (45) | 2013 | N/A |
| Trudeau et al. (46) | 2014 | 715 |
| Ye et al. (47) | 2020 | 29 |
| Tinay et al. (48) | 2016 | 3,774 |
| Hu et al. (49) | 2015 | 10 |
RARUN, robotic-assisted radical nephroureterectomy; N/A, not available.
De Groote et al. examined 78 patients with UTUC who underwent RARNU over a 10-year period using the da Vinci Si and Xi robotic systems (41). This study found that RARNU was safe and feasible with mean blood loss of 124 mL, operative time of 167 minutes, and average length of postoperative hospitalization of 4 days (41). Furthermore, after RARNU, patients had a 2- and 4-year RFS of 63% and 53%, respectively, and a 2- and 4-year OS of 79% and 66%, respectively (41). There has, therefore, been a growing interest into whether RARNU is superior to laparoscopic RNU, which remains controversial given cost-difference considerations.
Several studies have found that the short- and long-term oncological outcomes of RARNU were comparable to those of open and laparoscopic RNU (42,43). Conversely, a systematic review by Veccia et al. examining 10,155 RARNU and 31,093 laparoscopic RNU patients found that RARNU had less blood loss, lower risk of blood transfusions, and overall reduced postoperative complication rates compared to laparoscopic RNU (34). An analysis by Pathak et al. and Lee et al. also found a similar reduction in operating room time, blood loss, and length of stay after RARNU compared to laparoscopic RNU for UTUC (15,44) although Ribal et al. reported that laparoscopic RNU was non-inferior to RARNU with regard to RFS and CSS (45). Despite the benefits of RARNU, the authors noted that few studies have systematically examined the oncological benefits of RARNU compared to other RNU procedures.
Trudeau et al. compared short-term outcomes and costs between RARNU and laparoscopic RNU in 1,914 patients with UTUC (46). On multivariate analysis, patients undergoing RARNU were less likely to experience complications compared to patients who underwent laparoscopic RNU, but the robotic approach was associated with substantially higher costs (46). The study was also limited by the lack of adjustment for tumor stage and grade.
In a subsequent study by Ye et al. examining 29 RARNU patients versus 131 laparoscopic RNU patients in the treatment of UTUC, the authors showed no difference in 5-year intravesical RFS (88.0% vs. 85.5%) or distant metastasis-free survival (93.1% vs. 96.7%) although RARNU had a lower 5-year retroperitoneal RFS (77.3% vs. 87.7%) and CSS (71.2% vs. 84.7%) compared to laparoscopic RNU (47). The authors believed this discrepancy occurred because of an increased risk of local tumor spillage using the robotic platform although a major limitation of this study was the discrepancy in sample size between groups. In response, Tinay et al. performed a large, 10-year randomized trial of open, laparoscopic, and RARNU procedures for UTUC (48). This study reported no differences in perioperative outcomes between 13,317 laparoscopic and 3,774 RARNU patients (48). Furthermore, operative times and overall costs were higher in the laparoscopic and RARNU group compared to open RNU procedures (48).
Hu et al. performed a pair-matched analysis controlling for confounders with 18 laparoscopic and RARNU patients treated for UTUC which showed that RARNU patients had less blood loss, improved oral intake, and shorter hospital stay versus laparoscopic RNU patients (49). Unlike previous studies, RARNU patients actually had increased pain around their incision sites compared to laparoscopic RNU patients and oncological outcomes for RARNU were non-inferior to laparoscopic RNU with comparable operative, postoperative, and functional outcomes (49).
Overall, RARNU shows promise for reducing postoperative complications and improving outcomes for UTUC. Given the higher financial cost, however, it is unclear whether these improved outcomes offset the increased price tag associated with the procedure.
Bladder cuff excision
Several different methods have been described to resect the intramural ureter and bladder cuff around the ureteral orifice during open, laparoscopic, or RARNU. These include extravesical, transvesical, and endoscopic techniques. The extravesical approach involves circumferentially isolating and dissecting the entire intramural ureter and with gentle upward counter-traction on the ureter, the distal ureter is resected with its bladder cuff taking care to avoid injuring the contralateral ureter or ureteral orifice. The transvesical approach involves creating an anterior cystostomy along the anterior wall of the bladder, confirming the contralateral ureteral orifice, and circumferentially incising the ipsilateral ureteral orifice through the full thickness of the bladder wall including the mucosa and underlying detrusor muscle. The anterior cystostomy is subsequently closed in two layers after the intramural ureter and bladder cuff excision. The endoscopic approach involves placing the patient in the dorsal lithotomy position and subsequently using a resectoscope to incise a circumferential 10-mm cuff of bladder mucosa around the ureteral orifice. The resection site is then deepened down to the perivesical fat and the intramural ureter detached via a plucking maneuver from outside the bladder lumen. The specimen with the distal ureter and cuff of bladder is removed en-bloc during RNU.
Open excision (extravesical or transvesical approach)
With the open excision technique of the bladder cuff, the patient is placed in a supine position following a nephrectomy whereby a modified Pfannenstiel or Gibson incision is performed (50). Subsequently, the lower portion of the ureter is clamped, dissected, and removed along with the bladder cuff, which is secured extravesically or through an anterior cystotomy incision transvesically. This dissection, as mentioned above, can be done in two primary methods: extravesical or transvesical.
With an extravesical approach, the intramural ureter is dissected circumferentially to access the bladder while a transvesical approach involves opening the anterior bladder wall to visualize the ureteral orifice directly (50). During either approach, it is important not to block the contralateral ureteral orifice while performing extravesical or transvesical dissection and clamping of the distal ureter and resection of the bladder cuff. The remaining bladder is then sutured using in two layers (i.e., double-layer closure) to close the bladder mucosa, detrusor muscle layer, and bladder serosa in a water-tight fashion. This technique also allows easy recovery of the specimen for accurate histological examination (50). When comparing the open extravesical and transvesical methods, the transvesical technique allows for precise distal ureter and bladder cuff excision. Some studies have reported that an open approach to bladder cuff excision can reduce bladder tumor recurrence by 10.5% after RNU for UTUC (51). Nanigian et al., on the other hand, showed a lower risk of bladder tumor recurrence using RARNU rather than laparoscopic RNU with a transvesical approach (16% vs. 30%, respectively) (52,53). Comparisons using different bladder cuff excision techniques during RNU for UTUC are difficult to make given the lack of randomized controlled studies utilizing different approaches.
Transurethral resection (pluck technique)
An alternative surgical approach to open excision of the bladder cuff is transurethral resection (i.e., pluck technique), which avoids a secondary lower abdominal incision. This technique is accomplished transurethrally with cystoscopy by either resecting the intramural ureter with a resectoscope loop or removing the ureteric orifice and intramural ureter using a Collin’s knife (50). The procedure resects the ureteric orifice down to the perivesical fat, which allows for easy removal or “plucking” of the distal ureter during RNU (50). Fragkoulis et al. compared open excision of the bladder cuff in 192 patients versus transurethral resection and pluck technique in 186 patients during RNU for UTUC (54). Although the total operative time was lower in the transurethral resection group, the duration of postoperative catherization after surgery was lower in the open bladder cuff excision group (54). Interestingly, there was no difference between open excision and transurethral resection during RNU in the long-term bladder cancer recurrence rate (24% vs. 27%, respectively) and overall CSS (54). Although safe for proximal ureteral or renal pelvis tumors, transurethral resection should not be performed on suspected UTUC that involves the lower ureter or the ureterovesical junction due to an increased risk of tumor seeding, bladder cancer recurrence, and positive surgical margins (50). Additionally, patients with a previous history of pelvic irradiation therapy or pelvic inflammatory conditions should be excluded from the transurethral resection and pluck technique due to higher risk of persistent urine leak from poor healing of the bladder mucosa. Finally, the transurethral resection technique for the bladder cuff has a reported higher rate of tumor spillage and retroperitoneal recurrence of UTUC, so transurethral resection should only be considered when RNU is being performed laparoscopically or through robotic-assistance to reduce the incidence of these complications (50).
McNeil et al. retrospectively showed that laparoscopic RNU with transurethral resection and pluck technique of the bladder cuff did not show a significant difference in CSS when compared to traditional open techniques (15.2 vs. 17 months, respectively) although transurethral resection was limited to patients with UTUC confined to the renal pelvis (55). Allard et al. examined the oncological outcomes between various bladder cuff excision techniques for UTUC including 61 patients who underwent transurethral incision with a Collin’s knife, 29 patients who underwent open extravesical resection, and 20 patients who underwent open intravesical resection through an anterior cystotomy incision (56). Bladder cancer recurrence rates were 32.8%, 27.6%, and 40%, respectively with similar rates of metastasis-free survival regardless of surgical technique (56).
Intussusception
Unlike the open excision and transurethral resection approach, the intussusception method of bladder cuff excision involves insertion of a bulb-tipped ureteral catheter endoscopically to direct the ureter downward toward the bladder (50). The ureter is subsequently divided above the level of the catheter, and the remaining distal ureteral stump is intussuscepted into the bladder lumen using retrograde traction as a resectoscope is used to excise the ureteral orifice (50). Similar to the transurethral resection technique, intussusception is contraindicated in patients with UTUC located at the lower ureter or the ureterovesical junction due to an increased risk of tumor seeding, bladder cancer recurrence, and positive surgical margins (50). This surgical approach, consequently, has a higher rate of incomplete excision (18.7%) compared to other techniques (57). Additionally, Clayman et al. reported a bladder cancer recurrence rate of 21% using the transurethral ureteral intussusception technique although clinical trials comparing methods head-to-head are lacking (58).
Laparoscopic/RARNU techniques (extravesical stapling)
In recent years, there has been an increased interest in the application of bladder cuff excision methods with laparoscopic or RARNU to improve oncological outcomes and reduce postoperative complications (50). Laparoscopic and RARNU involve laparoscopic dissection of the entire distal and intramural ureter with isolation and excision of a bladder cuff with extravesical stapling (58). Currently, the EndoGIA and LigaSure devices have been used to reduce operative times and maintain a closed urinary system to prevent tumor spillage (58). Other combinations of laparoscopic and RARNU techniques have also been described, which include use of a harmonic scalpel as well as repositioning the patient with or without undocking the robot to shorten the operative time without reducing exposure to the distal ureter (52,59,60). Although RARNU has the potential to improve the dexterity, precision, and control of the surgeon in handling the distal/intramural ureter and bladder cuff, lack of comparative clinic trials, concerns with cost, and technical requirements limit widespread adoption into clinical practice (50,57).
Lymphadenectomy
Regional LND plays an important prognostic role at the time of RNU in the treatment of high-risk UTUC. For patients with a high-grade tumor, large tumor burden, and/or possible local invasion, LND is advantageous in terms of improving staging accuracy. Regional LND includes renal hilar, paracaval, precaval, and retrocaval nodes for right-sided tumors of the renal pelvis, upper, and middle third of the ureter. For left-sided tumors of the renal pelvis, upper, and middle third of the ureter, LND includes the renal hilar, paraaortic, and preaortic nodes. For tumors of the lower third of the ureter, an ipsilateral, extended pelvic LND including obturator, internal iliac, external iliac, and common iliac nodes is recommended with presacral nodes included based on physician preference.
Kondo et al. reported that in pT3 or greater UTUC, the extent of LND has a significant impact on CSS in N0 cases (61). Roscigno et al. also observed longer RFS and CSS in N0 patients who had at least eight lymph nodes removed during RNU for UTUC although this survival benefit did not translate with the extent of LND in N+ patients (62). Although the therapeutic benefit of regional LND at the time of RNU for high-risk UTUC still remains controversial and unclear, there is increasing literature and evidence that shows important staging and prognostic benefits of LND at the time of RNU especially for muscle-invasive or locally-advanced disease (pT2 and above) (63,64) with current guidelines recommending regional LND when these cases are suspected (65).
Single postoperative bladder instillation
Intravesical recurrences of urothelial carcinoma in the bladder are common after surgical treatment for high-risk UTUC with RNU, LND, and bladder cuff excision, occurring in 22–47% of patients secondary to implantation from the primary tumor (66). It is hypothesized that preoperative carcinogen exposure in the urothelium and intraluminal seeding and implantation from the upper urinary tract before and during RNU contribute to the high levels of bladder tumor recurrence in UTUC patients (67). Risk factors for intravesical recurrence include previous history of bladder cancer, smoking history, tumor multifocality in the upper urinary tracts, primary tumor size and stage, margin status, completeness of bladder cuff excision, and presence of CIS (30,66).
A multi-institutional study by Xylinas et al. found that the overall incidence of bladder cancer recurrence in patients with UTUC after RNU was 35% (66). Lee et al. reported similar findings with a previous history of bladder cancer, tumor multifocality, concomitant CIS, and a laparoscopic approach for RNU observed as risk factors for intravesical tumor recurrence (68). A meta-analysis by Yuan et al. examining 12,000 patients with UTUC found that female gender, larger tumor size, advanced pathological tumor stage, and history of bladder cancer were significant risk factors for bladder cancer recurrence after RNU (69). Interestingly, a systematic review and meta-analysis by Marchion et al. of over 2,000 patients with UTUC found a strong association between diagnostic ureteroscopy and intravesical recurrence of UTUC after RNU (70).
The beneficial effects of postoperative bladder instillation therapy of a single dose of mitomycin C administered within 72 hours after RNU have been shown in multiple clinical trials as well as a meta-analysis resulting in a 52% risk reduction of bladder cancer recurrence within the first postoperative year (71-73). O’Brien et al. reported that a single postoperative dose of mitomycin C resulted in a risk reduction of bladder cancer recurrence by 11% in the first year with the number needed to treat to prevent one bladder tumor being nine (73). Intravesical instillation of gemcitabine has also shown to significantly reduce recurrence in low-grade, non-muscle invasive bladder cancer and could represent a significantly cheaper option with less toxicity to administer in the postoperative period after RNU for UTUC (74).
A 2019 Cochrane review concluded that a single-dose of intravesical chemotherapy (i.e., mitomycin C) administered within 1 week post-RNU for UTUC reduced the risk of bladder cancer recurrence compared to no instillation [hazard ratio (HR): 0.51] (67). After 12 months of follow-up, this resulted in 127 fewer bladder cancer recurrences per 1,000 participants. The authors believed that a high concentration of chemotherapy within the bladder lumen would destroy any circulating cells in the urine and prevent any potential tumor spillage leading to intravesical recurrence in the future (67). This study, however, did not report the risks or adverse events associated with post-RNU intravesical chemotherapy instillation nor did it stratify UTUC patients based on operative approach, pathologic stage, or method of bladder cuff excision (67). Further clinical trials, therefore, are needed to determine which combination of operative approach and post-RNU intravesical chemotherapy instillation is ideal based on UTUC tumor size, stage, and location.
Perioperative systemic therapy
Surgery alone for high-risk UTUC with RNU has an overall 5-year survival rate less than 50% with clinical understaging common in terms of tumor stage and nodal status due to lack of accurate cross-sectional imaging and limitations to endoscopic techniques for biopsy of the primary tumor site. Ascertainment of the depth of the primary upper tract tumor is also difficult during diagnostic ureteroscopy due to technical limitations. Systemic chemotherapy before (neoadjuvant) or after (adjuvant) surgery represents a potential multidisciplinary option to improve long-term oncological outcomes in high-risk UTUC (75). UTUC typically arises from the renal pelvis and/or ureters, which are derived from the mesonephric duct and contain a number of embryologic, anatomic, and biological features that make them susceptible to chemotherapy due to microsatellite instability and other substances within the extracellular matrix (75). Several clinical trials have examined the use of chemotherapy in combination with surgical resection with RNU for high-risk UTUC.
Adjuvant chemotherapy has the advantage of accurate pathological staging from the RNU specimen, preventing overtreatment of superficial disease. Seisen et al. analyzed the National Cancer Database in a large retrospective, observational study of 3,253 UTUC patients, and found an OS benefit of adjuvant platinum-based chemotherapy for patients with pT3/T4 and/or pN+ UTUC (76). In the phase III randomized trial of adjuvant chemotherapy vs surveillance in UTUC conducted by the UK National Cancer Research Institute (designated the POUT trial), the authors reported that adjuvant chemotherapy (gemcitabine and either cisplatin or carboplatin if patient glomerular filtration rate (GFR) was 30–49 mL/min) provided a DFS benefit of 51% (HR: 0.49) (77). Adjuvant chemotherapy, therefore, plays an important supplemental role in treating patients with high-risk and/or locally advanced UTUC.
Neoadjuvant chemotherapy prior to RNU for UTUC allows for broader use of systemic therapy due to the availability of two renal units since in patients with chronic kidney disease, the surgically-induced loss of one kidney via RNU may render them ineligible for systemic therapy due to poor overall renal filtration. Most regimens again involve use of gemcitabine and either cisplatin or carboplatin if patient GFR is 30–49 mL/min although some believe that carboplatin-based regimens are inferior oncologically for UTUC and just add to the delay to definitive surgical therapy. Advanced imaging, such as positron emission tomography (PET)/CT, may be able to better identify and categorize patients who would benefit from neoadjuvant chemotherapy, such as patients with microscopic nodal disease on imaging or extension outside the muscularis layer of the collecting system.
One prospective study showed a pathologic complete response rate of 14% after neoadjuvant chemotherapy and RNU for high-risk UTUC (4 out of 29 patients) (78). A recent systematic review and meta-analysis by Kim et al. in 2019 evaluated four retrospective, observational studies on 318 patients receiving neoadjuvant chemotherapy for high-risk UTUC prior to RNU (79). Compared to controls, neoadjuvant chemotherapy improved OS and CSS by 57% and 59%, respectively (79). The effect of neoadjuvant chemotherapy on pathological tumor downstaging was 0.21, indicating that UTUC patients who received it had a 4.76-fold higher probability of being node negative at the time of surgery compared to the control group, which underwent surgery alone (79). Unlike adjuvant chemotherapy with the POUT trial, there is no phase III randomized controlled trial of neoadjuvant chemotherapy followed by surgery (i.e., RNU) versus surgery alone in UTUC to evaluate oncological efficacy with level 1 evidence.
Checkpoint inhibitors (PD-1/PD-L1 inhibitors) have been growing in favor for use in urothelial carcinoma of the bladder but only pembrolizumab and atezolizumab have phase III randomized data published in UTUC (i.e., KEYNOTE 045 and IMVigor 211, respectively) with only 27% of the included population diagnosed with the disease (80). As these immunotherapeutic agents are increasingly utilized in cancer care, further trials will be targeted toward the UTUC subgroup specifically as current evidence is lacking.
Nephron-sparing surgery
Nephron-sparing surgery is not typically recommended for high-risk UTUC although it may be considered for patients with imperative indications including solitary kidney, bilateral disease, baseline chronic kidney disease, or poor surgical candidates (74). Ureteroscopy with laser ablation of tumors could be considered in this select population for local control although the recurrence rate is high with risk for possible progression to metastatic disease (80).
Conclusions
High-risk UTUC is typically managed surgically with RNU, LND, and bladder cuff excision either using an open, laparoscopic, or robotic-assisted approach. A single instillation of intravesical chemotherapy post-RNU can also help reduce bladder tumor recurrences in the postoperative period regardless of surgical technique. Neoadjuvant or adjuvant chemotherapy can further enhance oncological outcomes with this disease although there is a push toward neoadjuvant therapy due to loss of a renal unit and reduced renal function precluding some patients from adjuvant therapy. Further clinical trials are needed to examine the effects of immunotherapy in the multi-disciplinary treatment of this disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Simon P. Kim) for the series “Surgical Management of Genitourinary Malignancies” published in AME Medical Journal. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-2020-smgm-01/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-2020-smgm-01/coif). The series “Surgical Management of Genitourinary Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol 2000;164:1523-5. [Crossref] [PubMed]
- Cosentino M, Palou J, Gaya JM, et al. Upper urinary tract urothelial cell carcinoma: location as a predictive factor for concomitant bladder carcinoma. World J Urol 2013;31:141-5. [Crossref] [PubMed]
- Marshall S, Stifelman M. Robot-assisted surgery for the treatment of upper urinary tract urothelial carcinoma. Urol Clin North Am 2014;41:521-37. [Crossref] [PubMed]
- Seisen T, Granger B, Colin P, et al. A Systematic Review and Meta-analysis of Clinicopathologic Factors Linked to Intravesical Recurrence After Radical Nephroureterectomy to Treat Upper Tract Urothelial Carcinoma. Eur Urol 2015;67:1122-33. [Crossref] [PubMed]
- Chan TY. World Health Organization classification of tumours: Pathology & genetics of tumours of the urinary system and male genital organs. Urology 2005;65:214-5. [Crossref]
- Rouprêt M, Babjuk M, Compérat E, et al. European Guidelines on Upper Tract Urothelial Carcinomas: 2013 Update. Eur Urol 2013;63:1059-71. [Crossref] [PubMed]
- Sun M. Management of upper urinary tract urothelial carcinoma. Expert Rev Anticancer Ther 2010;10:1955-65. [Crossref] [PubMed]
- Stravodimos KG, Komninos C, Kural AR, et al. Distal ureterectomy techniques in laparoscopic and robot-assisted nephroureterectomy: Updated review. Urol Ann 2015;7:8-16. [Crossref] [PubMed]
- Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: A series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009;115:1224-33. [Crossref] [PubMed]
- Ni S, Tao W, Chen Q, et al. Laparoscopic Versus Open Nephroureterectomy for the Treatment of Upper Urinary Tract Urothelial Carcinoma: A Systematic Review and Cumulative Analysis of Comparative Studies. Eur Urol 2012;61:1142-53. [Crossref] [PubMed]
- Simone G, Papalia R, Guaglianone S, et al. Laparoscopic versus Open Nephroureterectomy: Perioperative and Oncologic Outcomes from a Randomised Prospective Study. Eur Urol 2009;56:520-6. [Crossref] [PubMed]
- Alothman KI, Mehmood S, Alzahrani HM, et al. Surgical and oncological outcome after laparoscopic versus open nephroureterectomy for non-metastatic, upper-tract urothelial carcinoma. A single-centre experience. Saudi Med J 2020;41:25-33. [Crossref] [PubMed]
- Kim TH, Hong B, Seo HK, et al. The Comparison of Oncologic Outcomes between Open and Laparoscopic Radical Nephroureterectomy for the Treatment of Upper Tract Urothelial Carcinoma: A Korean Multicenter Collaborative Study. Cancer Res Treat 2019;51:240-51. [Crossref] [PubMed]
- Kim SH, Song MK, Kim JK, et al. Laparoscopy versus Open Nephroureterectomy in Prognostic Outcome of Patients with Advanced Upper Tract Urothelial Cancer: A Retrospective, Multicenter, Propensity-Score Matching Analysis. Cancer Res Treat 2019;51:963-72. [Crossref] [PubMed]
- Lee H, Kim HJ, Lee SE, et al. Comparison of oncological and perioperative outcomes of open, laparoscopic, and robotic nephroureterectomy approaches in patients with non-metastatic upper-tract urothelial carcinoma. PLoS One 2019;14:e0210401. [Crossref] [PubMed]
- Nazzani S, Bazinet A, Preisser F, et al. Comparison of perioperative outcomes between open and minimally invasive nephroureterectomy: A population-based analysis. Int J Urol 2019;26:487-92. [Crossref] [PubMed]
- Nouralizadeh A, Tabatabaei S, Basiri A, et al. Comparison of Open Versus Laparoscopic Versus Hand-Assisted Laparoscopic Nephroureterectomy: A Systematic Review and Meta-Analysis. J Laparoendosc Adv Surg Tech A 2018;28:656-81. [Crossref] [PubMed]
- Liu F, Guo W, Zhou X, et al. Laparoscopic versus open nephroureterectomy for upper urinary tract urothelial carcinoma: A systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e11954. [Crossref] [PubMed]
- Zhang S, Luo Y, Wang C, et al. Long-term oncologic outcomes of laparoscopic nephroureterectomy versus open nephroureterectomy for upper tract urothelial carcinoma: a systematic review and meta-analysis. PeerJ 2016;4:e2063. [Crossref] [PubMed]
- Hanske J, Sanchez A, Schmid M, et al. A Comparison of 30-Day Perioperative Outcomes in Open Versus Minimally Invasive Nephroureterectomy for Upper Tract Urothelial Carcinoma: Analysis of 896 Patients from the American College of Surgeons-National Surgical Quality Improvement Program Database. J Endourol 2015;29:1052-8. [Crossref] [PubMed]
- Sugihara T, Yasunaga H, Yu C, et al. Perioperative Outcome Comparisons Between Open and Laparoscopic Nephroureterectomy Among a Population-Based Cohort from 2010 to 2012. J Endourol 2015;29:770-6. [Crossref] [PubMed]
- Hanna N, Sun M, Trinh QD, et al. Propensity-score-matched comparison of perioperative outcomes between open and laparoscopic nephroureterectomy: a national series. Eur Urol 2012;61:715-21. [Crossref] [PubMed]
- Walton TJ, Novara G, Matsumoto K, et al. Oncological outcomes after laparoscopic and open radical nephroureterectomy: results from an international cohort. BJU Int 2011;108:406-12. [Crossref] [PubMed]
- Kitamura H, Maeda T, Tanaka T, et al. Comparison of laparoscopic, hand-assisted, and open surgical nephroureterectomy. JSLS 2014;18:288-93. [Crossref] [PubMed]
- Ariane MM, Colin P, Ouzzane A, et al. Assessment of oncologic control obtained after open versus laparoscopic nephroureterectomy for upper urinary tract urothelial carcinomas (UUT-UCs): results from a large French multicenter collaborative study. Ann Surg Oncol 2012;19:301-8. [Crossref] [PubMed]
- Zou L, Zhang L, Zhang H, et al. Comparison of post-operative intravesical recurrence and oncological outcomes after open versus laparoscopic nephroureterectomy for upper urinary tract urothelial carcinoma. World J Urol 2014;32:565-70. [Crossref] [PubMed]
- Blackmur JP, Stewart GD, Egong EA, et al. Matched-pair analysis of open versus laparoscopic nephroureterectomy for upper urinary tract urothelial cell carcinoma. Urol Int 2015;94:156-62. [Crossref] [PubMed]
- Kido K, Hatakeyama S, Fujita N, et al. Oncologic outcomes for open and laparoscopic radical nephroureterectomy in patients with upper tract urothelial carcinoma. Int J Clin Oncol 2018;23:726-33. [Crossref] [PubMed]
- Liu JY, Dai YB, Zhou FJ, et al. Laparoscopic versus open nephroureterectomy to treat localized and/or locally advanced upper tract urothelial carcinoma: oncological outcomes from a multicenter study. BMC Surg 2017;17:8. [Crossref] [PubMed]
- Peyronnet B, Seisen T, Dominguez-Escrig JL, et al. Oncological Outcomes of Laparoscopic Nephroureterectomy Versus Open Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: An European Association of Urology Guidelines Systematic Review. Eur Urol Focus 2019;5:205-23. [Crossref] [PubMed]
- Miyazaki J, Nishiyama H, Fujimoto H, et al. Laparoscopic Versus Open Nephroureterectomy in Muscle-Invasive Upper Tract Urothelial Carcinoma: Subanalysis of the Multi-Institutional National Database of the Japanese Urological Association. J Endourol 2016;30:520-5. [Crossref] [PubMed]
- Lim SK, Shin TY, Rha KH. Current status of robot assisted laparoscopic radical nephroureterectomy for management of upper tract urothelial carcinoma. Curr Urol Rep 2013;14:138-46. [Crossref] [PubMed]
- Phillips CK, Taneja SS, Stifelman MD. Robot-Assisted Laparoscopic Partial Nephrectomy: The NYU Technique. J Endourol 2005;19:441-5. [Crossref] [PubMed]
- Veccia A, Antonelli A, Francavilla S, et al. Robotic versus other nephroureterectomy techniques: a systematic review and meta-analysis of over 87,000 cases. World J Urol 2020;38:845-52. [Crossref] [PubMed]
- Hu JC, Silletti JP, Williams SB. Initial Experience with Robot-Assisted Minimally-Invasive Nephroureterectomy. J Endourol 2008;22:699-704. [Crossref] [PubMed]
- Teo XL, Lim SK. Robot-assisted nephroureterectomy: current perspectives. Robot Surg 2016;3:37-48. [Crossref] [PubMed]
- Aboumohamed AA, Krane LS, Hemal AK. Oncologic Outcomes Following Robot-Assisted Laparoscopic Nephroureterectomy with Bladder Cuff Excision for Upper Tract Urothelial Carcinoma. J Urol 2015;194:1561-6. [Crossref] [PubMed]
- Eandi JA, Nelson RA, Wilson TG, et al. Oncologic outcomes for complete robot-assisted laparoscopic management of upper-tract transitional cell carcinoma. J Endourol 2010;24:969-75. [Crossref] [PubMed]
- Pugh J, Parekattil S, Willis D, et al. Perioperative outcomes of robot-assisted nephroureterectomy for upper urinary tract urothelial carcinoma: a multi-institutional series. BJU Int 2013;112:E295-300. [Crossref] [PubMed]
- Yang CK, Chung SD, Hung SF, et al. Robot-assisted nephroureterectomy for upper tract urothelial carcinoma: the Taiwan Robot Urological Surgery Team (TRUST) experience. World J Surg Oncol 2014;12:219. [Crossref] [PubMed]
- De Groote R, Decaestecker K, Larcher A, et al. Robot-assisted nephroureterectomy for upper tract urothelial carcinoma: results from three high-volume robotic surgery institutions. J Robot Surg 2020;14:211-9. [Crossref] [PubMed]
- Lim SK, Shin TY, Kim KH, et al. Intermediate-term outcomes of robot-assisted laparoscopic nephroureterectomy in upper urinary tract urothelial carcinoma. Clin Genitourin Cancer 2013;11:515-21. [Crossref] [PubMed]
- Mullen E, Ahmed K, Challacombe B. Systematic review of open versus laparoscopic versus robot-assisted nephroureterectomy. Rev Urol 2017;19:32-43. [PubMed]
- Pathak RA, Hemal AK. Techniques and Outcomes of Robot-assisted Nephro-ureterectomy for Upper Tract Urothelial Carcinoma. Eur Urol Focus 2018;4:657-61. [Crossref] [PubMed]
- Ribal MJ, Huguet J, Alcaraz A. Oncologic outcomes obtained after laparoscopic, robotic and/or single port nephroureterectomy for upper urinary tract tumours. World J Urol 2013;31:93-107. [Crossref] [PubMed]
- Trudeau V, Gandaglia G, Shiffmann J, et al. Robot-assisted versus laparoscopic nephroureterectomy for upper-tract urothelial cancer: A population-based assessment of costs and perioperative outcomes. Can Urol Assoc J 2014;8:E695-701. [Crossref] [PubMed]
- Ye H, Feng X, Wang Y, et al. Single-docking robotic-assisted nephroureterectomy and extravesical bladder cuff excision without intraoperative repositioning: The technique and oncological outcomes. Asian J Surg 2020;43:978-85. [Crossref] [PubMed]
- Tinay I, Gelpi-Hammerschmidt F, Leow JJ, et al. Trends in utilisation, perioperative outcomes, and costs of nephroureterectomies in the management of upper tract urothelial carcinoma: a 10-year population-based analysis. BJU Int 2016;117:954-60. [Crossref] [PubMed]
- Hu CY, Yang CK, Huang CY, et al. Robot-Assisted Laparoscopic Nephroureterectomy versus Hand-Assisted Laparoscopic Nephroureterectomy for Upper Urinary Tract Urothelial Carcinoma: A Matched Comparison Study. Biomed Res Int 2015;2015:918486. [Crossref] [PubMed]
- Stravodimos KG, Komninos C, Kural AR, et al. Distal ureterectomy techniques in laparoscopic and robot-assisted nephroureterectomy: Updated review. Urol Ann 2015;7:8-16. [Crossref] [PubMed]
- Klingler HC, Lodde M, Pycha A, et al. Modified laparoscopic nephroureterectomy for treatment of upper urinary tract transitional cell cancer is not associated with an increased risk of tumour recurrence. Eur Urol 2003;44:442-7. [Crossref] [PubMed]
- Nanigian DK, Smith W, Ellison LM. Robot-Assisted Laparoscopic Nephroureterectomy. J Endourol 2006;20:463-5; discussion 465-6. [Crossref] [PubMed]
- Giannakopoulos S, Toufas G, Dimitriadis C, et al. Laparoscopic transvesical resection of an en bloc bladder cuff and distal ureter during nephroureterectomy. ScientificWorldJournal 2012;2012:658096. [Crossref] [PubMed]
- Fragkoulis C, Pappas A, Papadopoulos GI, et al. Transurethral resection versus open bladder cuff excision in patients undergoing nephroureterectomy for upper urinary tract carcinoma: Operative and oncological results. Arab J Urol 2017;15:64-7. [Crossref] [PubMed]
- McNeill SA, Chrisofos M, Tolley DA. The long-term outcome after laparoscopic nephroureterectomy: a comparison with open nephroureterectomy. BJU Int 2000;86:619-23. [Crossref] [PubMed]
- Allard CB, Alamri A, Dason S, et al. The method of bladder cuff excision during laparoscopic radical nephroureterectomy does not affect oncologic outcomes in upper tract urothelial carcinoma. World J Urol 2013;31:175-81. [Crossref] [PubMed]
- Viprakasit DP, Macejko AM, Nadler RB. Laparoscopic nephroureterectomy and management of the distal ureter: a review of current techniques and outcomes. Adv Urol 2009;2009:721371. [Crossref] [PubMed]
- Clayman RV, Garske GL, Lange PH. Total nephroureterectomy with ureteral intussusception and transurethral ureteral detachment and pull-through. Urology 1983;21:482-6. [Crossref] [PubMed]
- Hemal AK, Stansel I, Babbar P, et al. Robotic-assisted Nephroureterectomy and Bladder Cuff Excision Without Intraoperative Repositioning. Urology 2011;78:357-64. [Crossref] [PubMed]
- Park SY, Jeong W, Ham WS, et al. Initial experience of robotic nephroureterectomy: a hybrid-port technique. BJU Int 2009;104:1718-21. [Crossref] [PubMed]
- Kondo T, Nakazawa H, Ito F, et al. Impact of the extent of regional lymphadenectomy on the survival of patients with urothelial carcinoma of the upper urinary tract. J Urol 2007;178:1212-7; discussion 1217. [Crossref] [PubMed]
- Roscigno M, Shariat SF, Margulis V, et al. The extent of lymphadenectomy seems to be associated with better survival in patients with nonmetastatic upper-tract urothelial carcinoma: how many lymph nodes should be removed? Eur Urol 2009;56:512-8. [Crossref] [PubMed]
- Kondo T, Tanabe K. The role of lymph node dissection in the management of urothelial carcinoma of the upper urinary tract. Int J Clin Oncol 2011;16:170-8. [Crossref] [PubMed]
- Seisen T, Shariat SF, Cussenot O, et al. Contemporary role of lymph node dissection at the time of radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol 2017;35:535-48. [Crossref] [PubMed]
- Oosterlinck W, Solsona E, van der Meijden AP, et al. EAU guidelines on diagnosis and treatment of upper urinary tract transitional cell carcinoma. Eur Urol 2004;46:147-54. [Crossref] [PubMed]
- Xylinas E, Colin P, Audenet F, et al. Intravesical recurrence after radical nephroureterectomy for upper tract urothelial carcinomas: predictors and impact on subsequent oncological outcomes from a national multicenter study. World J Urol 2013;31:61-8. [Crossref] [PubMed]
- Hwang EC, Sathianathen NJ, Jung JH, et al. Single-dose intravesical chemotherapy after nephroureterectomy for upper tract urothelial carcinoma. Cochrane Database Syst Rev 2019;5:CD013160. [Crossref] [PubMed]
- Lee CH, Ku JY, Jeong CW, et al. Predictors for Intravesical Recurrence Following Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A National Multicenter Analysis. Clin Genitourin Cancer 2017;15:e1055-61. [Crossref] [PubMed]
- Yuan H, Chen X, Liu L, et al. Risk factors for intravesical recurrence after radical nephroureterectomy for upper tract urothelial carcinoma: a meta-analysis. Urol Oncol 2014;32:989-1002. [Crossref] [PubMed]
- Marchioni M, Primiceri G, Cindolo L, et al. Impact of diagnostic ureteroscopy on intravesical recurrence in patients undergoing radical nephroureterectomy for upper tract urothelial cancer: a systematic review and meta-analysis†. BJU Int 2017;120:313-9. [Crossref] [PubMed]
- Fang D, Li XS, Xiong GY, et al. Prophylactic intravesical chemotherapy to prevent bladder tumors after nephroureterectomy for primary upper urinary tract urothelial carcinomas: a systematic review and meta-analysis. Urol Int 2013;91:291-6. [Crossref] [PubMed]
- Ito A, Shintaku I, Satoh M, et al. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP Monotherapy Study Group Trial. J Clin Oncol 2013;31:1422-7. [Crossref] [PubMed]
- O'Brien T, Ray E, Singh R, et al. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial). Eur Urol 2011;60:703-10. [Crossref] [PubMed]
- Spiess PE, Agarwal N, Bangs R, et al. Bladder Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1240-67. [Crossref] [PubMed]
- Gregg RW, Vera-Badillo FE, Booth CM, et al. Perioperative chemotherapy for urothelial carcinoma of the upper urinary tract: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2018;128:58-64. [Crossref] [PubMed]
- Seisen T, Krasnow RE, Bellmunt J, et al. Effectiveness of Adjuvant Chemotherapy After Radical Nephroureterectomy for Locally Advanced and/or Positive Regional Lymph Node Upper Tract Urothelial Carcinoma. J Clin Oncol 2017;35:852-60. [Crossref] [PubMed]
- Birtle AJ, Lewis R, Johnson M, et al. Time to define an international standard of postoperative care for resected upper urinary tract transitional cell carcinoma (TCC) - opening of the peri-operative chemotherapy versus surveillance in upper tract urothelial cancer (POUT) Trial. BJU Int 2012;110:919-21. [Crossref] [PubMed]
- Margulis V, Puligandla M, Trabulsi EJ, et al. Phase II Trial of Neoadjuvant Systemic Chemotherapy Followed by Extirpative Surgery in Patients with High Grade Upper Tract Urothelial Carcinoma. J Urol 2020;203:690-8. [Crossref] [PubMed]
- Kim DK, Lee JY, Kim JW, et al. Effect of neoadjuvant chemotherapy on locally advanced upper tract urothelial carcinoma: A systematic review and meta-analysis. Crit Rev Oncol Hematol 2019;135:59-65. [Crossref] [PubMed]
- Leow JJ, Liu Z, Tan TW, et al. Optimal Management of Upper Tract Urothelial Carcinoma: Current Perspectives. Onco Targets Ther 2020;13:1-15. [Crossref] [PubMed]
Cite this article as: Kopel J, Sharma P. Narrative review of the surgical management of high-risk upper tract urothelial carcinoma. AME Med J 2021;6:17.

