
Utilizing a neurosurgical hydrogel sealant (DuraSeal sealant) for the closure of bronchopleural fistulas: a case report of a novel technique
Introduction
Many different bronchoscopic modalities are available for treatment of a bronchopleural fistulas although no comparative trials exist to identify a preferable technique. As we attempt to find more innovative and less invasive interventions for the closure of bronchopleural fistulas, we present a case report and discussion on the utility of DuraSeal® glue as a viable intervention for the closure bronchopleural fistulas. We present the following case in accordance with the CARE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-20-104/rc).
Case presentation
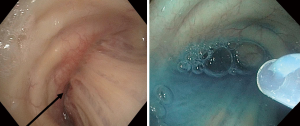
We present a case of a 60-year-old gentleman with history of stage IB (T2aN0M0) non-small cell lung cancer who underwent a right lower lobectomy. The postoperative recovery was complicated by a right pneumothorax that initially resolved with small bore chest tube drainage. Several days after discharge he re-presented with recurrent pneumothorax and subcutaneous emphysema. A right-sided chest tube was placed and found to have persistent air leak (PAL). Bronchoscopy was performed with general anesthesia and on positive pressure. Airway exam showed an intact right lower lobe (RLL) stump with a visible suture, but no visible defect or pitting mucosal changes. No consistent air leak was seen during positive pressure ventilation despite large pre-procedural PAL. The suction port of the bronchoscope was then attached to 8 liters per minute oxygen flow. Insufflation of the RLL stump induced an air leak confirming the site of the bronchopleural fistula (BPF). A 2.0 mm guide sheath was introduced via the working channel. Subsequently the two components of DuraSeal glue were instilled at the RLL stump site with adequate adherence visually. The air leak was no longer inducible with instillation of oxygen in the right lower lobe stump. No intraoperative complications were noted. The previously placed chest tube was converted to a Heimlich valve and the patient was safety discharged home. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The publication of this manuscript is waived from patient informed consent according to the ethics committee or institutional review board.
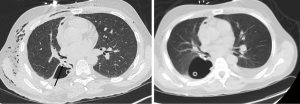
A CT chest 5 days after endoscopic intervention revealed a right loculated pleural effusion requiring antibiotics, exploratory thoracotomy, and placement of 2 chest tubes. He was discharged 5 days post thoracotomy with antibiotics and Heimlich valve. No evidence of glue failure was seen from time of glue instillation until discharge. Three months post procedure, he reports improvement in his symptoms and physical findings, with no signs of air leak. He continues to be followed outpatient with improvement in imaging (Figure 1).
Discussion
A PAL is defined as a BPF that lasts longer than 5–7 days (1,2). Specifically, lobectomy and pneumonectomy pose a high risk for development of a PAL via dehiscence of the bronchial stump (3,4). It is believed that a decrease in blood supply or damage to the bronchial stump, chemotherapy, radiation, and right sided surgery are risk factors for PAL (5). The presence of the fistula permits the sterile pleural space to be contaminated by endobronchial bacterial flora, leading to pleural space infections. As a result, morbidity and mortality remain high for this condition (3,4). Treatment often involves a multimodal approach including infection prophylaxis with antibiotics, chest tube for drainage of the pleural space, in addition to further surgical or bronchoscopic interventions (6).
Once localization and confirmation of the air leak is accomplished, the challenge becomes occluding the area successfully. Less invasive modalities are favorable compared to surgical intervention, especially given that most of these patients are already status post major thoracic surgery and are often poor surgical candidates (7). Many different bronchoscopic modalities are available for treatment of a BPF although no comparative trials exist. Main strategies for closing a small surgical stump defect include inducing scar tissue via laser, local silver nitrate application, ethanol, and electrocautery; versus applying adhesives including fibrin, albumin, glutaraldehyde, and acrylic. Larger defects have been closed by inserting implants such as metal coils, Watanabe spigots, endobronchial valves, or atrial-septal defect (ASD) closure devices. Placing gel foam, cellulose, antibiotics, or spongy calf bone to fill the defect has also been used in the past (1,7,8). A greater endoscopic success rate with utilization of adhesives is seen in fistulas under 5mm in diameter, with the highest success rates described when treating defects between 1–3 mm. Larger fistulas (greater than 8 mm), are preferably managed with stents, ASD closure devices, or surgery (6).
DuraSeal® (Integra Life Sciences, Plainsboro, NJ) is a synthetic, absorbable polyethylene glycol (PEG) hydrogel, historically used for the purpose of being a sealant and preventing cerebrospinal fluid leakage in cranial and spinal dural repair. Its inherent hydrophilic properties allow for the glue to swell after applied to the directed area. These properties permit the glue to provide a watertight seal, allowing more time (4–6 weeks) for healing. Neurosurgical studies have shown a high rate of successful intraoperative watertight seals with low rates of adverse effects in patients requiring dural closures (9,10).
DuraSeal® has been used in vascular and thoracic surgery for years, having achieving a certification mark in Europe for thoracic surgery in 2005 to prevent PAL from visceral pleura or airway injuries (11). We implemented the use of this glue endobronchially to treat a small stump leak. DuraSeal® requires the assistance of a disposable catheter (either Swan-Ganz, cut Fogarty balloon, or simple guide sheath) passed through the working channel of the bronchoscope. The sealant is easily delivered through the catheter but is initially in a liquid form as two separate components. The components harden and seal when mixed, therefore, they need to be delivered separately via these disposable catheters to the target airway to avoid damage of the bronchoscope (Figure 2). There is no need to traumatize the airway as is required with fibrin based products.
DuraSeal® sealant provided our patients with an innovative and less surgical intervention for the closure of their BPF. It also prevented the use of more invasive methods such as EBV’s and surgical intervention in our case. Non-surgical management such as the glue may provide added comfort for the patient while achieving a higher level of cost effectiveness for hospitals. Further studies are required to assess the long-term efficacy of the glue.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-20-104/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-104/coif).DMD reports personal fees from Boston Scientific, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The publication of this manuscript is waived from patient informed consent according to the ethics committee or institutional review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lazarus DR, Casal RF. Persistent air leaks: a review with an emphasis on bronchoscopic management. J Thorac Dis 2017;9:4660-70. [Crossref] [PubMed]
- Liberman M, Muzikansky A, Wright CD, et al. Incidence and risk factors of persistent air leak after major pulmonary resection and use of chemical pleurodesis. Ann Thorac Surg 2010;89:891-7; discussion 897-8. [Crossref] [PubMed]
- Hollaus PH, Lax F, el-Nashef BB, et al. Natural history of bronchopleural fistula after pneumonectomy: a review of 96 cases. Ann Thorac Surg. 1997;63:1391-6; discussion 1396. [Crossref] [PubMed]
- Katoch CDS, Chandran VM, Bhattacharyya D, et al. Closure of bronchopleural fistula by interventional bronchoscopy using sealants and endobronchial devices. Med J Armed Forces India 2013;69:326-9. [Crossref] [PubMed]
- Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest 2005;128:3955-65. [Crossref] [PubMed]
- Cusumano G, Alifano M, Lococo F. Endoscopic and surgical treatment for bronchopleural fistula after major lung resection: an enduring challenge. J Thorac Dis 2019;11:S1351-6. [Crossref] [PubMed]
- Wood DE, Cerfolio RJ, Gonzalez X, et al. Bronchoscopic management of prolonged air leak. Clin Chest Med 2010;31:127-33. Table of Contents. [Crossref] [PubMed]
- Motus IY, Bazhenov AV, Basyrov RT, et al. Endoscopic closure of a bronchopleural fistula after pneumonectomy with the Amplatzer occluder: a step forward? Interact Cardiovasc Thorac Surg 2020;30:249-54. [PubMed]
- Kim KD, Wright NM. Polyethylene glycol hydrogel spinal sealant (DuraSeal Spinal Sealant) as an adjunct to sutured dural repair in the spine: results of a prospective, multicenter, randomized controlled study. Spine 2011;36:1906-12. [Crossref] [PubMed]
- Cosgrove GR, Delashaw JB, Grotenhuis JA, et al. Safety and efficacy of a novel polyethylene glycol hydrogel sealant for watertight dural repair. J Neurosurg 2007;106:52-8. [Crossref] [PubMed]
- DuraSeal(R) Sealant CE Mark For Thoracic Applications [Internet]. [cited 2020 May 11]. Available online: https://www.adhesivesandsealants.com/doc/durasealr-sealant-ce-mark-for-thoracic-applic-0001
Cite this article as: Pasricha VG, Hutchinson CT, DiBardino DM. Utilizing a neurosurgical hydrogel sealant (DuraSeal sealant) for the closure of bronchopleural fistulas: a case report of a novel technique. AME Med J 2020;5:42.

