Spindle cell schwannoma in thoracic spinal cord: case report
Introduction
Spindle cells can be found in a variety of organs that originate from the mesenchyme, such as fat, muscles, connective tissues, bone, and blood vessels. However, this type of pathologic finding is rarely seen in a spinal schwannoma, the most common intradural extramedullary (IDEM) tumor. Most of the spindle cell tumors that have been reported to date are associated with sarcoma (1) or carcinoma (2,3) rather than schwannoma, and a case of a spinal schwannoma has not reported thus far. This case is being introduced because these non-typical schwannomas can show different clinical courses than normal benign schwannomas. We present the following case in accordance with the CARE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-20-14/rc).
Case presentation
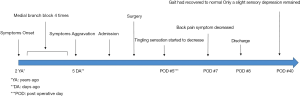
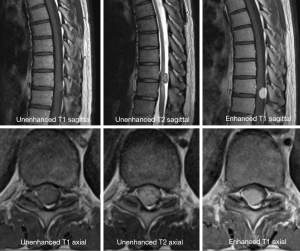
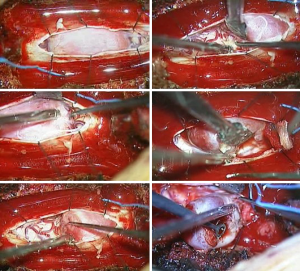
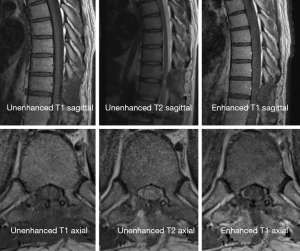
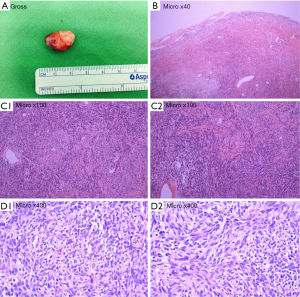
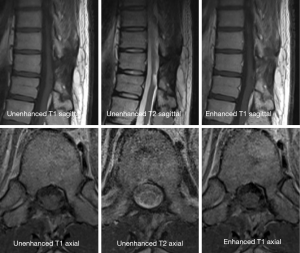
A 38-year-old man with lower back pain and a tingling sensation in both legs visited the Chungdam Wooridul Spine Hospital. The patient had no specific medical, familial, genetic, and psycho-social history. The symptoms started 2 years previously and were aggravated several days before the visit. He had already had medial branch block therapy 4 times at another hospital but did not show any improvement. Upon physical examination at the time of admission, his motor function was intact, but the patient showed a gait disturbance. He also showed hypoesthesia in both legs, especially below the L5 dermatome. Magnetic resonance imaging (MRI) showed a well-defined, ovoid-shaped IDEM mass, 17 mm × 11 mm × 9 mm, on the right dorsal side of the T10–11 level (Figure 1). The radiologic findings were suggestive of a schwannoma. Complete removal of the IDEM tumor was performed without neural damage. After general endotracheal anesthesia, the patient was placed in a prone position. Using lateral X-ray, the proper level was selected (T10/11) and opened in a minimally invasive fashion. A T10-11 total laminectomy and complete excision of the intradural tumor was performed (Figure 2). Postoperative MRI showed total removal of the IDEM mass and showed no remnant mass or abnormal fluid collection (Figure 3). A high signal still remained in the cord, which was considered to be a sign of compressive myelopathy. Upon cytologic examination, a well-circumscribed tumor mass was observed, and both the hypercellular portion and the loose edematous portion were shown together (Figure 4). At the high power field (×400), clusters of ovoid to spindle nuclei, without nuclear atypia mitotic features, were observed. The pathologic diagnosis was spindle cell schwannoma. One week post-operation, the patient’s back pain disappeared, and the tingling sensation resolved gradually by the 5th day after surgery (Figure 5). There weren’t any unanticipated events during recovery period, such as hematoma or post-operative swelling. Forty days post-operation, the patient’s gait had recovered to normal, and only a slight sensory depression remained. After 2 years of follow-up, the patient’s condition improved, and the tumor had not recurred on MRI at 15 months post operation (Figure 6).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
This report describes a case of a spindle cell schwannoma found in the thoracic spinal cord. Spinal schwannoma is relatively common and accounts for one-third of all primary spinal tumors (4) and 25% of adult intradural cord tumors (5,6). However, most schwannomas are benign, and less than 1% progress to malignancy.
Most typical schwannomas are hard, encapsulated neoplasms, composed of neoplastic Schwann cells. The histologic features of schwannomas include a high cellularity and the lack of Antoni B-type areas (7), and spindle cells are typically not found in schwannomas. Spindle cells are usually separate, rather than adherent or fusiform, and with indistinct cell borders. The nuclei are often fusiform as well, and their cytoplasmic tails may fade into the background. It may be possible to determine the tissue of origin, if there is any evidence of collagen, cartilage, bone, fat, or myxomatous material formation by the tumor cells (8).
There are some papers that are related to the increase of spindle cells in schwannomas. In these papers, this type of change is thought to suggest a malignant change. Endo et al. (9) reported a case of a spindle cell-type malignant schwannoma in peripheral nerve sheath tumors that originated from the soft tissue of the patient’s lower back. Chung et al. (10) also reported a similar case, which was expressed in the form of intraosseous metastasis. In both cases, the spindle cell-type, malignant, peripheral nerve sheath tumor, which led to a benign schwannoma, was reported to be very rare with very aggressive progression and a poor prognosis. In addition, spindle cell tumors that originate from cells other than schwannoma have also been reported in most cases to be associated with sarcoma or carcinoma, and their prognoses were also poor. Arnesen et al. (1) reported spindle cell tumors of the thoracic spinal cord, which was diagnosed as a spindle cell sarcoma that originated from a uterine leiomyosarcoma. Lewis et al. (2) reported spindle cell carcinomas of the head and neck, and Terada (3) reported spindle cell carcinomas of the lung. In this case, most of the patients showed a poor prognosis. Although most schwannomas are benign, it is important to not misdiagnose spindle cells as a lesion of the cellular type of schwannomas, because they can be an important clue for malignant progression (11). In this case, surgery was performed relatively early; a total resection was performed, and there was no evidence of recurrence during the 2 years of follow-up thereafter.
Conclusions
Spindle cell schwannoma is a rare lesion, and this case represents the first report of this type of tumor, presenting in the thoracic spinal cord. In this case, the tumor has not recurred 2 years after the early surgical treatment. Due to the poor prognosis compare to other type of schwannomas, aggressive surgical treatment and pathologic diagnosis should be prioritized.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-20-14/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-14/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arnesen MA, Jones JW. Spindle cell neoplasm of the thoracic spine. Ultrastruct Pathol 1992;16:29-34. [Crossref] [PubMed]
- Lewis JS Jr. Spindle cell lesions--neoplastic or non-neoplastic?: spindle cell carcinoma and other atypical spindle cell lesions of the head and neck. Head Neck Pathol 2008;2:103-10. [Crossref] [PubMed]
- Terada T. Spindle cell carcinoma of the lung: Frequency, clinical features, and immunohistochemical studies of three cases. Respiratory Medicine CME 2010;3:241-5. [Crossref]
- Nittner K. Spinal meningiomas, neurinomas and neurofibromas and hourglass tumors. Handbook of Clinical Neurology 1976;20:177-322.
- Celli P, Trillò G, Ferrante L. Spinal extradural schwannoma. J Neurosurg Spine 2005;2:447-56. [Crossref] [PubMed]
- McCormick PC, Post KD, Stein BM. Intradural extramedullary tumors in adults. Neurosurg Clin N Am 1990;1:591-608. [Crossref] [PubMed]
- Jeon JH, Hwang HS, Jeong JH, et al. Spinal schwannoma; analysis of 40 cases. J Korean Neurosurg Soc 2008;43:135-8. [Crossref] [PubMed]
- Coles EH. Veterinary clinical pathology. vol 3rd edition. WB Saunders, 1980.
- Endo M, Yamamoto H, Harimaya K, et al. Conventional spindle cell-type malignant peripheral nerve sheath tumor arising in a sporadic schwannoma. Hum Pathol 2013;44:2845-8. [Crossref] [PubMed]
- Chung JY, Kim SS, Kim SK. Spindle cell type malignant peripheral nerve sheath tumor arising in benign schwannoma with multiple intraosseous spinal metastasis: A case report. J Back Musculoskelet Rehabil 2017;30:1129-35. [Crossref] [PubMed]
- Rodriguez FJ, Folpe AL, Giannini C, et al. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol 2012;123:295-319. [Crossref] [PubMed]
Cite this article as: Kim SJ, Shin SH, Lee SH, Bae J. Spindle cell schwannoma in thoracic spinal cord: case report. AME Med J 2020;5:43.