
Urinary diversion and reconstruction following radical cystectomy for bladder cancer: a narrative review
Introduction
The first urinary diversion was described in 1852 by John Simon, who created bilateral ureterosigmoidal fistulas in a 13 year old child with bladder exstrophy (1). Since that time, there has been drastic expansion in the diversity of urinary diversions available to patients, and their primary application has expanded from children with neurogenic bladder and congenital anomalies to patients undergoing surgical reconstruction after radical cystectomy for bladder cancer. Here we intend to describe and compare urinary diversion techniques of historical and current significance through comprehensive review of the breadth of the peer reviewed literature, and furthermore review future perspectives and areas of active research in urinary reconstruction after radical cystectomy.
We present the following article in accordance with the Narrative Review reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-20-76/rc).
Oncologic indications for radical cystectomy
Bladder cancer is the 6th most common malignancy in the United States, with 80,470 new cases and 17,670 deaths from bladder cancer in 2019 (2). While the majority of bladder cancers are low grade and stage, and are able to be treated in a minimally invasive fashion, high grade bladder cancer can be extremely aggressive, and extirpative surgery is indicated for many patients with more severe disease in the form of radical cystectomy. While there are numerous guidelines produced around the world that provide treatment algorithms for the management of bladder cancer, it is generally agreed that radical cystectomy is indicated for all patients with clinically localized muscle invasive bladder cancer, that is those with T2 or greater disease with no definitive evidence of nodal or distant metastatic disease (3-6). These guidelines also advise that radical cystectomy should be considered in patients with concerning variant histology, high grade T1 with concerning features such as CIS, and in those with BCG failure, defined as persistent or recurrent CIS within twelve months of completion of appropriate BCG therapy, recurrent Ta/T1 within 6 months of finishing adequate BCG therapy, and persistent high grade T1 at the first evaluation after the initiation of BCG (5-10). In these patients who undergo radical cystectomy, choice of urinary diversion that minimizes morbidity and maximizes quality of life is paramount.
Cutaneous ureterostomy
After removal of the urinary bladder, one potential option for urinary diversion is to bring the distal ends of the ureters to the skin to form bilateral cutaneous ureterostomies. While this represents the simplest and least invasive method of urinary reconstruction after radical cystectomy, its use is now limited due to very high rates of stricture and associated complications, and it is primarily of historical interest. In the current era, cutaneous ureterostomy is typically only utilized in situations of desperation related to severe patient comorbidities that limit use of bowel for urinary reconstruction. Indeed, in historical series of cutaneous ureterostomy for reconstruction after radical cystectomy, stricture rates as high as 69% have been reported, although many of these patients received neoadjuvant radiation, which was typical at the time (11).
More contemporary series have worked to develop new methods of cutaneous ureterostomy that hope to reduce its morbidity and make it a viable option for patients who are poor candidates for other diversions. A retrospective review of 310 patients at the University of South Florida with a median follow-up of 25 months who underwent cutaneous ureterostomy from 1996 to 2010, primarily after radical cystectomy, demonstrated an overall rate of ureteral obstruction of only 13.2%. They cited a technique in which they are able to minimize rate of stricture through utilization of a wide abdominal wall hiatus and lateral spatulation of the ureters. Additionally, they used appendiceal interposition when needed to take tension off the left ureter, and with prolonged stenting greater than 3 months post-operatively, rates of stricture were as low as 4.5% (12).
Bowel segments used for urinary reconstruction
Stomach
The use of stomach for urinary reconstruction after radical cystectomy was pioneered by Leong and colleagues at Queen Mary’s Hospital in Hong Kong in the 1960’s and 1970’s (13), and despite its drawbacks and the rarity of its use, remains an important part of a urologists armamentarium in select situations. Stomach is particularly advantageous for use in urinary reconstruction in patients with short gut syndrome, so as to not further sacrifice intestinal absorption, in patients with severe adhesive disease, as the stomach tends to be less involved than other gastrointestinal structures, and in patients who cannot tolerate reabsorption of urinary solutes, such as those with renal insufficiency (13). Both continent and incontinent diversions can be fashioned from stomach, in the form of stomach conduits, orthotopic neobladders, and continent cutaneous diversions (13-15). One study of eight patients that underwent gastric neobladder formation after radical cystectomy revealed significantly worse incontinence at 63% compared to the ileal or ileocecal neobladder groups at 8% to 23% (P=0.02), and a high rate of reoperation at 38% (16). Urodynamic parameters were also significantly worse, with reduced bladder capacity and compliance (16). Another study examined the long-term consequences of the use of stomach for urinary reconstruction in 29 children, and found that 51.7% of patients had significant complications including hematuria-dysuria syndrome and loss of bladder compliance requiring intervention. Most concerningly, De Novo gastric adenocarcinoma formed in the bladders of three of the children that underwent gastroplasty within 15 years of their surgeries, and all three died of metastatic disease (17). Indeed, while use of stomach for urinary diversion and reconstruction confers numerous advantages in selected patients with contraindications to use of other segments of bowel for reconstruction, such as sensitivity to metabolic derangements and severe intrabdominal adhesions, for a large majority of patients the drawbacks outweigh the potential benefits.
Jejunum
While it is technically feasible to use jejunum for urinary diversion, and it is used for this purpose in rare circumstances, the severe metabolic complications associated with this segment of bowel, and its distinct lack of advantages compared to the use of ileal segments, highly limit its use. Use of jejunum in urinary diversion is associated with a severe hypochloremic, hyponatremic, hyperkalemic metabolic acidosis, as well as a significant azotemia, related to the resorptive affinity of the jejunal mucosa to urinary solutes (18,19). While some more contemporary studies have posited lower rates of electrolyte imbalance (20), given the availability of other segments of bowel and the lack of advantages associated with use of jejunum, we would advise against the use of jejunum for urinary reconstruction except when there is no other suitable conduit for urinary diversion.
Ileum
Throughout most of the world, the use of ileum, particularly in the form of ileal conduit urinary diversions, has become the mainstay of urinary reconstruction after radical cystectomy. The ileum has a number of qualities that make it an excellent segment of bowel for urinary reconstruction. It is readily available, comprising 2/5 of the overall length of small bowel, and most segments can be easily mobilized to the pelvis to facilitate complex reconstructive procedures. It is of a small caliber, which makes it ideal for conduit creation and ureteral replacement, but can also be readily constructed into reservoirs with excellent urodynamic parameters (21,22). In contrast with jejunum, use of ileum for urinary diversion is associated with a hyperchloremic, hypokalemic, metabolic acidosis, and these metabolic abnormalities tend to be much less severe, facilitating construction of large urinary reservoirs with longer segments of bowel. In one study of low pressure ileal bladder substitutes, only 3 of 100 patients required long term sodium bicarbonate substitution for metabolic acidosis, and all of those patients had reservoirs constructed from >50cm of ileum. Furthermore, No patients required nutritional supplementation or had any other notable metabolic derangements (23). Another series of 200 ileal neobladders found similar results, with mild temporary acidosis occurring in 47% of patients, and only 3% having metabolic abnormalities that merited hospitalization (24). Use of ileum for conduit formation leads to even less metabolic complications, as the urine is in contact with bowel for a more limited period of time and over a smaller surface area. Overall, ileum is an excellent segment of bowel to use for urinary diversion, as it functions well as both a conduit and urinary reservoir, is easily accessible and utilized, and carries low risk of metabolic abnormalities and malabsorptive issues.
Ileocolic
Ileocolic segments, that is segments that include the terminal ileum, ileocecal valve, and proximal colon, are also used for a number of urinary diversions, as they take advantage of the distinct anatomy of this region, and in particular the ileocecal valve, which can be used as a component of continence and anti-reflux mechanisms. Use of ileocecal segments for diversion carries a higher risk of diarrhea and metabolic and nutritional abnormalities relative to other bowel segments due to resection of the terminal ileum and ileocecal valve, which are critical for absorption of some nutrients and regulation of passage of stool into the colon (25). In a study of 94 patients who underwent the ileocecal Mainz Pouch I procedure who were followed for at least five years, there were no symptomatic metabolic abnormalities, however, based on laboratory findings, 32% of patients required B12 supplementation, 37% of patients required treatment for metabolic acidosis, and 32% of patients reported stool frequency (26). Despite elevated risks of metabolic derangement, malabsorption, and diarrhea, ileocecal segments are also a safe and viable portion of bowel to utilize for urinary reconstruction.
Colon
While less popular than ileum, colonic segments are also commonly used for urinary diversion, and carry distinct advantages. Much of the colon, like the ileum, is easily mobilized into the pelvis. While it is of a larger caliber than the ileum, it can still be used for both ureteral substitution and conduit creation, and can also be used to construct effective urinary reservoirs for continent diversion such as a sigmoid pouch. Colonic segments, like ileum, tend to have minimal metabolic consequences that are easily managed, and like ileum the abnormalities are characterized by a hyperchloremic metabolic acidosis. Nutritional deficiencies are not expected with use of pure colon segments for diversion, as it does not play a significant role in absorption of nutrients, although patients with colonic diversions, particularly continent diversions that use longer segments, have been shown to have issues with diarrhea that can affect long term quality of life (27). For patients that have a history of pelvic radiation, transverse colon, by virtue of its fixation in the upper abdomen by the hepatocolic ligament proximally and the phrenocolic distally, is typically spared from damaging radiation (28). The sigmoid colon has a particular application for diversion in patients undergoing pelvic exenteration, especially in those with gynecologic malignancy, sparing the patient from needing a bowel anastomosis as their stool is diverted proximally (29). Colonic segments also allow for more effective and technically easier construction of anti-reflux mechanisms at the ureteroenteric anastomosis relative to small bowel. Overall, colon is a great option for construction of both urinary conduits and continent urinary reservoirs, with a similar risk profile to ileum.
The characteristics of different segments of bowel used in urinary diversion are summarized in Table 1.
Table 1
| Intestinal Segment | Example diversions | Advantages | Associated electrolyte disturbance | Other complications/disadvantages | Typical utilization |
|---|---|---|---|---|---|
| Stomach | Conduit, Gastric Pouch | Least absorption of urinary compounds, less bacteriuria, produces less mucus | Hypochloremic, hypokalemic metabolic alkalosis | Hematuria-dysuria syndrome with ureteral and cutaneous ulcerations, intestinal ulceration when using antral segments, dumping syndrome, iron deficiency, vitamin B12 deficiency. | Patients with severe adhesions and/or patients with history of pelvic radiation. Short gut syndrome. Renal insufficiency |
| Jejunum | Conduit | None. Only used when no other option is available due to severe metabolic derangements | Severe hypochloremic, hyponatremic, hyperkalemic metabolic acidosis | Reabsorption of urea leading to azotemia, malabsorption | Patients in whom no other segment of bowel is available for diversion |
| Ileum | Conduit, Kock Pouch, T pouch, Double-T pouch, Studer Neobladder, Hautmann Neobladder, Ghoneim Neobladder, Camey Neobladder (I and II) | Easily mobile to the pelvis, small diameter, less metabolic abnormalities, good urodynamic properties | Hyperchloremic, hypokalemic, metabolic acidosis | Vitamin B12 deficiency, often in pelvic radiation fields. | Most commonly used segment of bowel for urinary reconstruction when available |
| Ileocolic | Mainz Pouch/Neobladder, Indiana Pouch, Florida Pouch, Miami Pouch, Penn Pouch, Right Colon Pouch, Le Bag Neobladder | Easily mobilized, ileocecal valve and appendix can be used to create help create continence and anti-reflux mechanisms | Hyperchloremic, hypokalemic, metabolic acidosis | Vitamin B12 deficiency, fat malabsorption and diarrhea due to loss of regulation from ileocecal valve | Construction of urinary reservoirs, particular for continent cutaneous diversion using the ileocecal valve and/or appendix to construct the continence mechanism |
| Colon | Conduit (Transverse and Sigmoid), Sigmoid Pouch | Less bowel obstructions, easily mobilized, least metabolic and malabsorptive consequences, transverse segment is spared from pelvic radiation, easier to construct anti-reflux anastomosis | Hyperchloremic, hypokalemic, metabolic acidosis | Large caliber can require tapering, significant mucus production, diarrhea | Patients with history of pelvic radiation (transverse). Patients undergoing pelvic exenteration (sigmoid). Surgeon preference |
The different intestinal segments that can be utilized for urinary diversions, including their applications, advantages, and drawbacks.
Ureterointestinal anastomosis
The topic of whether to perform a refluxing or non-refluxing anastomosis when constructing urinary conduits and reservoirs with bowel has been the subject of debate for years, and each approach carries its own set of advantages and disadvantages. In the past, it was theorized that a non-refluxing anastomosis, although somewhat more technically challenging to construct, would lead to reduced rates of renal deterioration due to lower rates of infection and nephrolithiasis (30). A study was carried out in the dog model wherein dogs received an ileal conduit with a refluxing anastomosis for one of their kidneys and a non-refluxing colon conduit for the other. This study demonstrated evidence of pyelonephritis and renal scarring in 83% of kidneys connected to ileal conduits with a refluxing anastomosis and only 7% of kidneys connected to colon conduits with a non-refluxing anastomosis (30). Similar results were found in human studies, with a Swedish cohort demonstrating evidence of renal scar on renal scintigraphy in 11 of 17 kidneys with a refluxing ureterointestinal anastomosis and in only 6 of 18 kidneys with an anti-refluxing anastomosis (P=0.06) (31).
However, the clinical relevance of this has been brought into question as despite decreased rates of renal scar, there was no difference in GFR between refluxing and non-refluxing anastomosis in the Swedish cohort (32), and another study demonstrated minimal deterioration of renal function and acceptable rates of pyelonephritis in a long term study of patients with refluxing ileal conduit urinary diversions (33). Concern has also been raised that some non-refluxing anastomosis techniques may lead to unacceptable rates of ureteroenteric stricture relative to refluxing anastomotic techniques (34-37). Refluxing anastomoses also have the advantage of permitting the reflux of contrast into the collecting system, aiding in surveillance of upper tract recurrence in high risk patients. Given the above, we perform a refluxing ureterointestinal anastomosis for most urinary diversions at our center, and this preference is shared with other high volume centers (38,39). Regardless of the technique that is utilized to perform the ureterointestinal anastomosis, certain principles should be applied to minimize the incidence of complications such as stricture and urine leak, including a tension free anastomosis, rigorous preservation of the ureteral blood supply, and utilization of silastic stents across the ureterointestinal anastomosis (40).
The primary methods used to establish a refluxing ureteroileal anastomosis are the Bricker and Wallace techniques. The Bricker technique was one of the first methods described to perform an ureteroileal anastomosis, and has withstood the test of time due to its simplicity and effectiveness. In the modern iteration of the technique, each ureter is separately anastomosed to the bowel segment in an end-to-side fashion with absorbable sutures (41). A similar technique can also be used to create refluxing ureterocolonic anastomoses (42). In contrast to the Bricker approach, the Wallace technique is an end-to-end anastomosis wherein the two ureters are spatulated and anastomosed together in a side to side fashion before being anastomosed to the end of the bowel segment as one unit (43). The choice of technique between these two approaches is largely up to surgeon preference and experience, with the available literature reporting no significant difference in terms of ureteroenteric stricture rate between the two methods (44,45).
There are a number of methods that are used to establish a non-refluxing ureterointestinal anastomosis, both for colonic and small bowel segments. The full spectrum of techniques used to perform a nonrefluxing ureterointestinal anastomosis is outside of the scope of this article, however there are certain common principles to these techniques. The initial non-refluxing anastomotic techniques were those building off Coffey’s method (46) that utilized tunneling of the ureter into the submucosa and taenia of the large bowel for use in ureterosigmoidostomy, and were later applied to both conduit urinary diversions and continent diversions (47,48). A number of different methods for performing ureterointestinal anastomosis using this principle have since been devised, both in large (49,50), and small bowel (51). Ureteral nipple mechanisms can also be constructed to create an anti-reflux anastomosis (52), as can inserting redundant length of the ureter into the bowel segment and fixing it to the mucosa (53) or seromuscular layer of the bowel wall (54).
Incontinent urinary diversion using bowel segments
The only incontinent urinary diversion that uses bowel segments for reconstruction after radical cystectomy is a urinary conduit, wherein the ureters are anastomosed to a segment of bowel proximally, and distally the bowel is matured to a cutaneous urostomy that passively drains into a stoma appliance. Conduit urinary diversions are the most popular urinary diversions throughout much of the world for reconstruction after radical cystectomy (55,56), and are valued for their reliability, effectiveness, and relative simplicity when compared to other urinary diversions. As described above, stomach (13), jejunum (18), ileum (41), and colon (29) can all be utilized to create a urinary conduit, and each segment of bowel carries unique advantages and disadvantages in this application, although ileal segments are most commonly used.
While ileal conduit urinary diversion is a safe, simple, and effective option for urinary diversion, and has become the most popular diversion in most of the world, all forms of urinary diversion after radical cystectomy carry a high risk of acute and long term complications. A study of 214 patients from MD Anderson who underwent radical cystectomy with ileal conduit urinary diversion demonstrated an operative mortality rate of 3.3%, with early complications in 27.6% of patients, including wound infection in 10.7% of patients and dehiscence in 5.6%, in addition to other common perioperative complications such as PE and pneumonia. It is important to consider, however, that many of those patients received neoadjuvant pelvic radiation, which was typical at the time (57). In terms of long term complications, a study of 131 patients with greater than 5 years of follow-up demonstrated an overall complication rate of 66%, including issues with: renal function in 27%, the stoma in 24%, UTIs in 23%, the ureterointestinal anastomosis in 14%, and urolithiasis in 9%. The rate of complications increased by a great deal over time, and was 94% in patients more than 15 years out from surgery (58). Parastomal hernias in particular represent a significant challenge to the urologist, as they are relatively common, with some studies reporting an incidence as high as 65%, and their repair is quite challenging, carrying significant morbidity and risk of recurrence (59). Prophylactic use of surgical mesh at the time of ostomy creation, either synthetic or biologic, has been posited as a potential strategy to prevent the development of parastomal hernias, and has been well studied in the general surgery literature for traditional ostomies with relatively good results (60). Recently, a few groups have evaluated the use of prophylactic mesh specifically in ileal conduits to prevent the formation of parastomal hernias, with a notable study from Memorial Sloan Kettering demonstrating a 56% relative risk reduction in parastomal hernias with prophylactic mesh compared to historical controls (P=0.043) (60). In practice, it is likely that the use of prophylactic mesh at the time of ileal conduit creation will be limited to a large degree by concerns about complications related to the mesh, and especially by a lack of familiarity and experience in the use of surgical mesh and parastomal hernia repair among urologists in much of the world.
Continent urinary diversions
Continent urinary diversions are urinary reservoirs created after bladder removal that aim to store urine without significant leakage, and allow for intermittent bladder emptying via the urethra, a catheterizable channel, or the anus. The utilization of continent urinary diversions differs greatly by geographic region and institution, with a recent analysis showing that rates of continent diversions are less than 15% overall in the US and Sweden, and as high as 75% at specialized institutions (55). Indeed, the technical difficulty of performing continent diversions and cultural factors that impact patient decision making lead to vastly different choices among urinary diversions. It is also of the utmost importance that one very carefully selects the patients to whom they offer continent urinary diversion. All patients who receive continent urinary diversion must have the motivation, manual dexterity, and mental capacity to intermittently catheterize and irrigate their urinary reservoir at regular intervals, have sufficient bowel length to allow for harvesting of long segments of ileum and colon for diversion without nutritional compromise, and have the renal function to withstand increased reabsorption of urinary solutes relative to conduit urinary diversions due to increased dwelling time of urine in the reservoir and increased surface area of bowel utilized.
Regardless of whether patients choose an orthotopic, continent cutaneous, or incontinent diversion, ultimate outcomes in quality of life are relatively similar between the different reconstructive strategies. Indeed, a large study from Vanderbilt University compared health related quality of life outcomes between ileal conduit and neobladder, and after adjusting for age, the data was suggestive of a small difference in favor of neobladder, but this was not statistically significant (P=0.09) (61). Furthermore, a recent metanalysis of 21 studies comparing neobladder and ileal conduit in terms of quality of life scores showed no difference in 16 studies, favored neobladder in 4 studies, and favored ileal conduit in 1 study (62). While there may be a small advantage to neobladder in terms of health related quality of life, there is little evidence to definitively support this. Overall, other than sexual dysfunction, which occurs at a high rate in all patients who undergo cystectomy, patients are largely satisfied and adapt well to life after cystectomy, with 97% reporting no difficulty with basic daily living activities (63).
Orthotopic urinary diversions
The goal of orthotopic urinary diversion after radical cystectomy is to most closely approximate normal urologic anatomy and function following bladder removal by fashioning a reservoir from a bowel segment that can store urine in a continent fashion at safe pressures and allow for volitional voiding to occur through the natural urethra. The first orthotopic urinary diversion to gain widespread acceptance was the Camey procedure, wherein a 40 cm segment of the distal ileum was arranged in a U-shaped configuration in the pelvis with a ureter anastomosed to the proximal and distal portions of the bowel and the urethra anastomosed to the midpoint between the two (64). While this procedure created a functional and usually continent urinary reservoir, high pressures within the reservoir due to use of a still tubularized ileal segment with intact peristalsis led to incontinence and concerns about upper tract deterioration. Modern orthotopic diversions therefore focus on creating a low pressure reservoir through detubularization of the bowel segment and rearrangement of the intestine in a way that inhibits coordinated peristalsis. Numerous reservoirs that utilize these principles have since been developed that are constructed from a number of bowel segments, including the ileal Studer Neobladder (65) and Hautmann W-Neobladder (66), the ileocolonic Mainz III Pouch (67) and Le Bag Pouch (68), and the purely colonic Reddy Sigmoid Pouch (69).
Orthotopic urinary diversion has been demonstrated to be very safe in appropriately selected patients, although like all forms of urinary diversion does carry significant risks. In a series of 923 patients undergoing ileal neobladder at the University of Ulm, complications were recorded in 40.8% of patients, including hydronephrosis in 16.9% of patients, hernia in 6.4%, SBO or ileus in 3.6% and febrile UTI in 5.7%, along with three deaths related to the urinary diversion (70).
Continent rectal diversions
From the first urinary diversion in 1852, until the advent of conduit urinary diversions in the 1950’s, continent rectal diversions were the dominant form of urinary diversion used throughout the world. These diversions avert the flow of urine such that its efflux can be modulated by the anal sphincter. Initially these diversions consisted of a simple ureterosigmoid anastomosis, which was fraught with issues with infection and sepsis due to reflux of stool into the collecting system. In the 1920’s, Coffey and colleagues realized that the high colonic pressures during bowel evacuation and subsequent ureteral reflux were contributing to sepsis and renal deterioration, and they described a tunneled anti-reflux anastomosis that made the ureterosigmoidostomy a much safer and more effective form of urinary diversion (46). A number of continent rectal diversions based on this principle were devised, with the goal of increasing the capacity and decreasing the pressure of the rectum to improve continence, decreasing reflux up to the kidneys (71-73), and even creation of an orifice for drainage of urine adjacent to but separate from the anus that utilizes the same sphincter mechanism (74). Due to an increased risk of development of colorectal malignancy with admixture of urine and stool in the colon (75), metabolic abnormalities (76), and concern for combined fecal and urinary incontinence, continent rectal diversions have largely been abandoned, even by the pioneers of the more advanced techniques at the University of Mansoura (55), and are primarily of historical interest.
Continent catheterizable cutaneous diversions
Continent cutaneous urinary diversions in one form or another have been utilized as early as 1950, when Gilcrest and Merricks described the assembly of a continent right colon pouch (77). Further study has led to the development of a plethora of continent cutaneous urinary diversions of increasing complexity and efficacy, with the goal of producing the ideal reservoir that can store large volumes of urine at low pressure, has a reliable continence mechanism that facilitates easy intermittent passage of a urinary catheter, and has acceptable perioperative and postoperative complication rates. Despite the numerous continent diversions that have been developed over the years, they share certain key principles. In contrast to orthotopic urinary diversions, the majority of continent catheterizable diversions, and especially those utilized in the modern era, use an ileocecal segment to construct the reservoir and continence mechanism (67,78). Only the catheterizable Kock Pouch (79), and variations that have been developed from it such as the double-T pouch (80) use purely ileal segments, and few perform these techniques due to the technical complexity of and complications associated with creation of the continence mechanisms.
The most critical aspect of continent cutaneous urinary diversion is the continence mechanism, as the specifications of its construction differentiates most of the diversions that have been developed. There are three categories that make up the majority of these continence mechanisms. The first is the creation of intussuscepted nipple valves through the telescoping and fixation of ileal segments, either without the support of the ileocecal valve, such as in the Kock Pouch (79) or T-Pouch (80), or using the ileocecal valve as a point of fixation, as in the more modern iterations of the Mainz Pouch (81). The direct use of the ileocecal valve by reinforcing it to produce a reliable continence mechanism, first demonstrated in the Indiana Pouch, is also commonly used, and is highly effective and easy to construct relative to nipple valves (78), making it very popular. The final group of continence mechanisms are those that take advantage of the Mitrofanoff principle, and use either appendix, first described in the Penn Pouch (82), or with tubularized cecum in patients who lack a usable appendix (83). Similar to orthotopic urinary diversion, it is critical that detubularized segments are used to increase capacity and decrease pressure within the reservoir, and methods such as ileal interposition and Heineke-Mikulicz closure of the pouch can be used to inhibit coordinated peristaltic contraction that could contribute to leakage (78).
While catheterizable diversions are an excellent option in well-selected patients, they do carry a significant risk of complications. One study of 129 patients that underwent Indiana pouch with a mean follow-up of 41.1 months found an 89.6% overall rate of complications, including leakage in 28.8% of patients, urolithiasis in 10.4%, and stomal stenosis in 15.2% (84).
Current research/future perspectives in urinary diversion
Much of the pioneering work in urinary diversion, as can be seen in this review, was carried out decades in the past, and practice patterns in urinary diversion have been relatively stable for quite some time at most high volume centers. While the diversions that we perform are largely the same, current areas of research such as minimally invasive urinary diversion, regenerative medicine, and enhanced recovery after surgery (ERAS) pathways have the potential to make these operations safer, less invasive, and expedite recovery.
Minimally invasive radical cystectomy with urinary diversion; intracorporeal and extracorporeal diversion
Great advances have been made in minimally invasive techniques for radical cystectomy since Gill described the first laparoscopic approach at the Cleveland Clinic in 2000 (85). The groundbreaking RAZOR trial, along with multiple meta-analysis, have shown that minimally invasive approaches tend to incur shorter hospital stays, less intraoperative blood loss, and less post-operative complications than open procedures (86-89), and recently published data reports oncologic outcomes comparable to open surgery (90). The debate over whether intracorporeal urinary diversion (ICUD) or extracorporeal urinary diversion (ECUD) following a minimally invasive radical cystectomy provides superior outcomes has come to the forefront of academic urology.
Several studies that directly compared outcomes of ICUD and ECUD following radical cystectomy are summarized in Table 2. The first published report was a 2010 case series by Pruthi et al., which found ICUD to have significantly longer operating time, but slight reductions in estimated blood loss (EBL), hospital stay, and post-operative bowel stasis (91). Larger studies have shown mixed results, with Ahmed and Lenfant reporting less perioperative blood loss and need for transfusion in ICUD, but Carrion reporting no difference in this parameter (92-95). It is important to note that the studies discussed above included patients undergoing reconstruction with both orthotopic neobladder and ileal conduit, and were classified solely by if they underwent intracorporal or extracorporeal diversion.
Table 2
| Study | Year | Study Type | Sample size (n) | Operative time (min) | Intraoperative blood loss (mL) | Hospitalization (days) | 30-days complications (%) | 90-day complications (%) |
|---|---|---|---|---|---|---|---|---|
| Zhang | 2020 | Retrospective review | ICUD:301; ECUD:375 | ICUD:396; ECUD:375 (P=0.001) | ICUD:300; ECUD:400 (P<0.001) | ICUD:6; ECUD:7 (P<0.001) | No difference | No difference |
| Lenfant | 2018 | Retrospective review | ICUD:74; ECUD:34 | No difference | Increased perioperative transfusion rate for ECUD | No difference | ICUD:47.3; ECUD:38.2 (P=0.4) | ICUD:18.9; ECUD:29.4 (P=0.2) |
| Carrion | 2019 | Retrospective review | ICUD:14; ECUD:10 | No difference | No difference | No difference | No difference | Higher rate of uretero-ileal and urethra-neobladder stricture in ECUD group |
| Ahmed | 2014 | Retrospective review | ICUD: 87; ECUD: 570 | No difference | IDUC: 375 EDUC:385 (P=0.758) EDUC: greater need for perioperative transfusion | ICUD: 9; ECUD:8 (P=0.086) | ICUD:35; ECUD:43 (P=0.07) | ICUD:41; ECUD:49 (P=0.055) |
| Pruthi | 2010 | Prospective case series | ICUD: 12; ECUD: 20 | ICUD:318 ECUD:252 (P=0.001) | ICUD:221; ECUD:266 (P=0.654) | ICUD: 4.5; ECUD:5.3 (P=0.393) | No difference | No difference |
ICUD versus ECUD ileal conduit following robotic-assisted radical cystectomy
Several studies have directly compared outcomes of intracorporeal versus extracorporeal ileal conduit creation following radical cystectomy and are summarized in Table 3.
Table 3
| Study | Year | Study type | Sample size (n) | Operative time (min) | Intraoperative blood loss (mL) | Hospitalization (days) | 30-day complications (%) | 90-day complications (%) |
|---|---|---|---|---|---|---|---|---|
| Tan | 2019 | Retrospective review | ICUD:59; ECUD: 68 | ICUD:300; ECUD:375 (P=0.019) | ICUD:300; ECUD: 425 (P=0.035) | No difference | ICUD: 48.4; ECUD:71.4 (P=0.008) | ICUD:15.2; ECUD:16.2 (P=0.878) |
| Bertolo | 2019 | Prospective controlled trial | ICUD:60; ECUD:66 | ICUD:420; ECUD: 360 (P=0.004) | No difference | No difference | ICUD:22; ECUD:13 (P=0.5) | ICUD:2; ECUD: 1 (P=0.7) |
| Hayn | 2011 | Prospective controlled trial | ICUD:37; ECUD:37 | ICUD:356; ECUD:390 (P=0.019) | No difference | No difference | No difference | No difference |
Hayn and colleagues first examined this topic in a prospective control trial in 2011, which found ICUD to have shorter operating time, but no difference in complications or blood loss (96). Interestingly, this trial found that patients who underwent ICUD had significantly better body image scores in the weeks following the procedure compared to those who underwent ECUD, likely due to fewer visible abdominal incisions. More recent studies by Bertolo and Tan have demonstrated mixed results in terms of how operating time and complication rates compare between intracorporal and extracorporeal ileal conduit (97,98).
Recently, new strategies for intracorporeal ileal conduit creation following RARC have been proposed. Kaouk and colleagues at the Cleveland Clinic demonstrated the viability of using a single-port robotic system for totally intracorporeal radical cystectomy and ileal conduit in a 2019 series of four patients (99). This study showed a mean total operating time of 7.5 hours, with no patients suffering intra or post-operative complications. Similar research from the Cleveland Clinic demonstrated the feasibility of a robotic, single-port, transperineal approach to ileal conduit creation in the cadaveric model following cystectomy (100).
ICUD and ECUD ileal conduit following laparoscopic-assisted radical cystectomy (LARC)
Given the prohibitively expensive nature of robotic surgery in many locales, LARC has gained increasing interest as a minimally invasive approach to the treatment of bladder cancer. Intracorporal ileal conduit reconstruction following LARC was first described in the literature by Haber in 2007, and showed ICUD to be extremely limited by technical difficulty and long operative times (101). However, the results of Kanno and colleagues’s trial in 2018 demonstrated promising advances in laparoscopic ICUD, with their technique reducing early post-operative complications and intraoperative blood loss (102). In a 2018 retrospective review, Wu and colleagues found intracorporeal diversion to reduce the time until return of bowel function, duration of postoperative hospital stays, and Clavien Grade II complications at 30 days postoperatively (103). Interestingly, Wu also found laparoscopic ICUD to increase mean lymph node yield and reduce total operative time. A more recent trial by Perlin et al. showed laparoscopic ICUD with late division of the ureters to have improved renal outcomes when compared to conventional ICUD laparoscopic approach without additional operating time required (104). Notably, Wang showed non-inferior oncological outcomes when comparing intracorporeal and extracorporeal ileal conduit creation following laparoscopic radical cystectomy (105). Table 4 summarizes the findings of published trials comparing laparoscopic ICUD and ECUD.
Table 4
| Study | Year | Study Type | Sample size (n) | Operative time (min) |
Intraoperative blood loss | Hospitalization | 30-Day Complications (%) | 90-Day Complications (%) |
|---|---|---|---|---|---|---|---|---|
| Wu | 2018 | Retrospective review | ICUD:19; ECUD:19 | ICUD:364; ECUD297 (P=0.007) | ICUD:393; ECUD:205 (P=0.252) | No significant difference | ICUD:22; ECUD:13 (P=0.002) | ICUD:12.9; ECUD:18.6 (P=0.014) |
| Wang | 2018 | Prospective controlled trial | ICUD:20; ECUD:25 | No significant difference | No significant difference | ICUD:11; ECUD:17 (P=0.001) | No significant difference | No significant difference |
| Kanno | 2018 | Prospective controlled trial | ICUD:35; ECUD:31 | ICUD:690 ECUD:540 (P=0.001) | ICUD:560; ECUD:1165 (P=0.001) | ICUD:41; ECUD:48 (P=0.073) | ICUD:39; ECUD:71 (P=0.016) | No significant difference |
| Haber | 2007 | Prospective case series | ICUD:8; ECUD:18 | ICUD:564; ECUD:378 (P=0.0001) | ICUD:788; ECUD:378 (P=0.0002) | N/a | ICUD:87; ECUD:28 (P=0.004) | ICUD:35; EDUC:17 (P=0.61) |
ICUD and ECUD orthotopic neobladder following radical cystectomy
The first totally intracorporeal robotic-assisted neobladder and radical cystectomy was performed by Beecken and colleagues at Germany’s Goethe University in 2003 (106). Despite this pioneering procedure being described over 15 years ago, the practice of total intracorporeal orthotopic neobladder remains rare given the technical complexity and long operating times involved. Further work by Goh and colleagues has shown increasing feasibility of total-intracorporeal neobladder creation following RARC, and it has been suggested that a total intracorporeal technique has potential to improve surgical outcomes compared to an extracorporeal approach (107).
Intracorporeal continent catheterizable pouch following radical cystectomy (Indiana Pouch)
Many recent advances have been made toward minimally invasive approaches to creation of continent cutaneous urinary diversions. Goh first described a robotic approach to creating a modified Indiana Pouch following a standard six-port robotic cystectomy in 2015 (108). Bowel mobilization, ureterocolic anastomosis, and creation of pouch opening via appendectomy are all performed intracorporeally, followed by stoma maturation through standard extracorporeal procedure. This innovative technique showed no major 90-day complications, and continued functionality at one year (108). Desai et al. of University of Southern California created a similar robotic procedure, described in a 10-patient case series published in 2017 (109). This procedure differs in that mobilization of the bowel is initiated at the midtransverse colon and proceeds proximally, rather than proceeding distally from the ileocolonic junction. In place of externalizing the stoma inferior and lateral to the umbilicus on the right, Desai’s procedure externalizes the stoma through the umbilical incision (109). Desai found similar results to Goh’s approach, with only one subject not having satisfactory pouch function at one year. Matulewicz and colleagues at Memorial Sloan Kettering also recently published the results of a series of 11 patients who underwent a robotic cystectomy and creation of continent catheterizable urinary pouch (110). This procedure required mean operative time of 8.5 hours, with only one patient having a Clavien 3 complication post-operatively and had excellent functional outcomes at three months.
Regenerative medicine and tissue engineering for urinary diversion
Atala and colleagues first showed the potential of autologous bladder grafts for use in augmentation cystoplasty in their 2006 trial published in The Lancet (111). Since this landmark study, the use of tissue engineering to generate autologous bladders for implantation following radical cystectomy has been at the forefront of urologic research. The current leading approach to creating a bioartificial bladder graft is through cell culture of urothelial and smooth muscle cells which are then seeded into a bladder scaffold. Debate has focused over whether biologically derived or acellular synthetic grafts provide superior results. Several in-vivo studies examining this topic are compared in Table 5 (112-116).
Table 5
| Study | Year | Study description | Scaffold material | Scaffold class | Major findings |
|---|---|---|---|---|---|
| Wang | 2019 | Rat model of partial bladder graft | Polyglycolic acid | Biologic | 1. Grafts successfully regenerated incised bladder |
| 2. Grafts showed observable histologic vascularization in-vivo | |||||
| Young | 2018 | Ovine model partial bladder graft comparing cellular and acellular scaffolds | Acellular dermal matrix and biological ovine-derived matrix | Biologic versus Synthetic comparison | 1. Ovine-derived biologic scaffold showed more properties resembling native tissue |
| 2. Acellular matrix did not integrate less into host tissue compared to biological matrix | |||||
| 3. Both biological and acellular matrix grafts showed similar functional performance at 3 months | |||||
| Shakhssalim | 2017 | Canine model of whole bladder graft after cystectomy | Poly (ε-caprolactone) poly(lactic acid) | Synthetic | 1. No leakage of implanted whole bladders noted at three month |
| 2. In-vitro cultured tissue was successfully implanted in-vivo | |||||
| Shi | 2017 | Canine model of partial bladder graft | Collagen-binding basic fibroblast growth factor acellular matrix | Synthetic | Grafted material successfully regenerated smooth muscle, nervous, and vascular tissue |
| Zhao | 2015 | Rat model of whole bladder grafts after cystectomy | Silk fibroin/Acellular matrix bilayer | Synthetic | 1. Subjects with whole-bladder graft had better bladder compliance, capacity, rate of success at 12 weeks compared to those with graft cystoplasty |
| 2. No local or systemic toxic response to whole bladder grafts were noted |
Notably, the first clinical trial investigating the use of autologous bioengineered urinary conduits in human subjects following radical cystectomy (NCT01087697) was initiated in 2010 under the direction of the regenerative medicine company Tengion Inc. Initial data has demonstrated the feasibility of regenerative medicine approaches, with urothelial tissue being identified in the bioengineering neo-conduit. Clinical data has not yet been officially released, but investigators have signaled that initial results are promising (117).
ERAS protocols for radical cystectomy
ERAS protocols provide a standardized, evidence-based approach to preparing a patient for surgery and guiding their post-operative recovery. This concept was first described in the colorectal surgery literature by Kehlet in 1997 and has rapidly spread in the field of Urology (118). While immense variability exists in the ERAS pathways utilized by different institutions, most share similar principles. In general, protocols are broken into three basic stages: pre-operative, operative, and post-operative. The design and results of several ERAS protocols for radical cystectomy are summarized in Table 6 (119-126).
Table 6
| Study | Year | Study design | Sample size | Preoperative practices | Intraoperative practices | Postoperative practices | Outcomes |
|---|---|---|---|---|---|---|---|
| Zhang | 2020 | Retrospective review | 185 | 1. Focused optimization of medical comorbidities, smoking and alcohol status | 1. Avoidance of excessive fluid infusion | 1. Chewing gum after surgery | 1. Decreased intraoperative blood loss |
| 2. Preoperative pneumatic compression | 2. Avoidance of intra-abdominal drains | 2. Clear liquid diet immediately post-operatively | 2. Earlier return of bowel function | ||||
| 3. Two days of carbohydrate loading before surgery | 3. Removal of NG tube after surgery | 3. Encouragement to stay out of bed for 6 hours day after surgery | 3. Earlier ambulation | ||||
| 4. Allowing clear liquids and solids 2 and 6 hours before surgery respectively | 4. Warming with heated IV fluid | 4. Early removal of pelvic drain | 4. Decreased incidence of pneumonia, DVT, urine leakage, GI obstruction | ||||
| 5. Reduced length of hospitalization | |||||||
| 6. Decreased 30-day readmission | |||||||
| Dunkman | 2019 | Retrospective review | 100 | 1. Pre-surgical education about procedure | 1. Transdermal amlodipine patch | 1. Scheduled adjunctive non-opioid analgesia with epidural | 1. Reduced length of hospitalization |
| 2. No use of preoperative bowel regimen | 2. Low thoracic epidural | 2. Alvimopan to restore bowel function | 2. Reduced time to first stool | ||||
| 3. Carbohydrate loading night before surgery | 3. Avoidance of IV opioids | 3. Discontinuation of IV fluids when oral diet achieved | 3. Reduction in overall costs | ||||
| 4. Oral analgesic loading with acetaminophen, gabapentin | 4. Intraoperative dexamethasone | 4. Encouragement to stay out of bed for 6 hours daily | |||||
| Pang | 2018 | Prospective control trial | 393 | 1. Counseling with stoma therapist, surgeon | 1. Ovarian sparing when possible in women | 1. Gum chewing after surgery to promote bowel function | 1. Fewer blood transfusions |
| 2. Exercise of one hour per day | 2. Avoidance of NG tube | 2. Mobilization day after surgery | 2. Reduced readmissions | ||||
| 3. Carbohydrate loading night before surgery | 3. Analgesia with rectus sheath block | 3. Reduced length of hospitalization | |||||
| Mukhtar | 2018 | Prospective control trial | 26 | 1. Carbohydrate loading night before surgery | 1. Avoidance of nasogastric tubes/drain | 1. Early enteral feeding | 1. Reduced hospital stay |
| 2. No oral bowel prep | 2. Mid-thoracic epidural analgesia | 2. Avoidance of opioid analgesia | 2. Early return to full oral diet | ||||
| 3. Avoidance of nasogastric tubes and drains | 3. Avoidance of excessive fluid infusion | 3. Mobilization the day after surgery | 3. No change in complication, mortality, readmission rates | ||||
| Tan | 2018 | Retrospective review | 250 | 1. No bowel prep | 1. Minimally-invasive, robotic procedure | 1. NG tube removal immediately after surgery | 1. Reduced hospital stay |
| 2. High-carbohydrate drink given night before and 6 hours before surgery | 2. Spinal anesthesia provided | 2. Scheduled acetaminophen with morphine only for breakthrough pain | 2. Lower 90-day readmission | ||||
| 3. Transesophageal doppler intraoperatively to goal-directed fluid management | 3. Metoclopramide and oral fluids immediately post-operatively | 3. Lower gastrointestinal complications in first 90 days | |||||
| 4. Diet first day post-operatively | |||||||
| 5. Mobilization first day post-operatively | |||||||
| Liu | 2018 | Retrospective review | 84 | 1. Smoking cessation, weight loss counseling | 1. Warming of patient with fluids and forced air | 1. Gum chewing after surgery to promote bowel function | 1. Reduced length of hospitalization |
| 2. No preoperative bowel prep | 2. Thoracic epidural anesthesia | 2. Oral nutrition same day as surgery | 2. No difference in readmission rates, complication rates | ||||
| 3. Solid foods up to 6 hours before surgery, liquids up to 2 hours | 3. Mobilization same day as surgery | ||||||
| 4. Encouragement of increased carbohydrate consumption | 4. Avoidance of NG tube | ||||||
| Wei | 2018 | Retrospective review | 91 | 1. Nutritional supplementation night before surgery 2. Encouragement of alcohol and smoking cessation | 1. Epidural analgesia with immediate post-operative rectus sheath block | 1. Encouragement of gum chewing after surgery | 1. Shorter time to first flatus, pelvic drain removal |
| 2. Minimal incision | 2. Encouragement of clear fluids day after surgery | 2. Reduced hospital costs | |||||
| 3. Patient-controlled opioid analgesia | 3. Reduced blood loss/transfusion | ||||||
| 4. Introduction of solid diet with first flatus | 4. Reduced post-operative complications | ||||||
| Semerjian | 2018 | Retrospective review | 56 | 1. No preoperative bowel prep | 1. Thoracic epidural placement with immediate postoperative rectus sheath block | 1. Continuation of alvimopan until return of bowel function | 1. Reduce cost of hospitalization |
| 2. 16-ounce Gatorade given 2 hours before surgery 3. Alvimopan given 1 hour before surgery | 2. Use of vacuum wound dressing | 2. Analgesia acetaminophen, ketorolac for breakthrough pain, no opioid analgesia | 2. Reduce length of hospitalization | ||||
| 3. Clear liquid diet started immediately post-operatively | 3. Reduced need for NG tube and parenteral nutrition | ||||||
| 4. Ambulation the night of surgery | 4. No difference in readmission or complication rates |
Pre-operative protocols tend to include counseling in order to assess patient expectations and address the lifestyle considerations involved with ostomy creation. Education also typically involves health status optimization through intensive exercise and nutritional counseling. At our institution, pre-operative patients participate in an in-person “stoma boot camp”, with the goal of maximizing patient adjustment to living with an ostomy, as well as decreasing postoperative stoma-related interventions and frustrations. Several studies have supported the practice of preoperative carbohydrate loading either the day before or day of surgery. This practice is thought to reduce post-operative insulin resistance, and has been shown to reduce hospital stay, opioid analgesic use, and time to return of bowel function (127).
Intraoperative practices have focused on instituting non-narcotic pain control in the perioperative period, minimally invasive surgical practices, and maintaining adequate normothermia throughout the procedure. Perioperative pain control is often achieved through thoracic epidural, rectus sheath block, or a combination of both. This practice has been shown to reduce opioid analgesic use and improve post-operative pain scores (128).
Postoperative practices typically include early mobilization, promotion of early restoration of bowel function, and reduction in opioid use. Protocols vary considerably on the timeframe in which mobilization is achieved, with some encouraging ambulation the same day as surgery and other prolonging up to 3 days. Use of wearable technology to monitor ambulation and vital signs in post-operative patients has been shown to be a viable strategy both for tracking and encouraging ambulation, and also for early identification of complications, both before and after discharge (129). Early return of bowel function is often pursued through early enteral feeding and treatment of bowel stasis with the mu-antagonist alvimopan. Most protocols seek to reduce opioid analgesia through scheduled use of acetaminophen, and ketorolac or other NSAIDs for breakthrough pain.
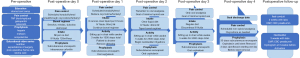
Our institution’s ERAS protocol mirrors many international trends, and is described in Figure 1.
Conclusions
There are a number of ways that urinary reconstruction after radical cystectomy can be accomplished that use a wide variety of techniques and substrates. While some procedures have clearly shown superiority relative to others, there is little high level evidence that compares the urinary diversions that receive widespread use in the modern era, and much of the choice of the urinary diversion that a patient receives is based on surgeon experience and patient preference. Future advances in the short term will likely be aimed at making the diversions that we already perform less invasive and improving peri-operative care. Ultimately, the hope is that one day we can obviate the need for intestinal segments, which clearly entail most of the morbidity of these operations, and engineer bespoke autologous urinary diversions.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Simon P. Kim) for the series “Surgical Management of Genitourinary Malignancies” published in AME Medical Journal. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-20-76/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-20-76/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-76/coif). The series “Surgical Management of Genitourinary Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pannek J, Senge T. History of urinary diversion. Urol Int 1998;60:1-10. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Chang SS, Bochner BH, Chou R, et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J Urol 2017;198:552-9. [Crossref] [PubMed]
- Alfred Witjes J, Lebret T, Comperat EM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol 2017;71:462-75. [Crossref] [PubMed]
- Kubota Y, Nakaigawa N. Committee for Establishment of the Clinical Practice Guideline for the Management of Bladder C, et al. Essential content of evidence-based clinical practice guidelines for bladder cancer: The Japanese Urological Association 2015 update. Int J Urol 2016;23:640-5. [Crossref] [PubMed]
- Chinese guidelines for diagnosis and treatment of urothelial carcinoma of bladder 2018 (English version). Chin J Cancer Res 2019;31:49-66. [Crossref] [PubMed]
- Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol 2016;196:1021-9. [Crossref] [PubMed]
- Babjuk M, Bohle A, Burger M, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol 2017;71:447-61. [Crossref] [PubMed]
- Steinberg RL, Thomas LJ, Mott SL, et al. Bacillus Calmette-Guerin (BCG) Treatment Failures with Non-Muscle Invasive Bladder Cancer: A Data-Driven Definition for BCG Unresponsive Disease. Bladder Cancer 2016;2:215-24. [Crossref] [PubMed]
- Administration UFaD. BCG-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for Treatment Guidance for Industry. US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER), 2018.
- Feminella JG Jr, Lattimer JK. A retrospective analysis of 70 cases of cutaneous ureterostomy. J Urol 1971;106:538-40. [Crossref] [PubMed]
- Rodríguez AR, Lockhart A, King J, et al. Cutaneous ureterostomy technique for adults and effects of ureteral stenting: an alternative to the ileal conduit. J Urol 2011;186:1939-43. [Crossref] [PubMed]
- Leong CH. Use of the stomach for bladder replacement and urinary diversion. Ann R Coll Surg Engl 1978;60:283-9. [PubMed]
- Bissada NK, Keane T, Caczmarek AT, et al. Experience with coapted gastric tube outlet and composite gastrointestinal reservoir for continent cutaneous urinary diversion. J Urol 2004;171:229-31. [Crossref] [PubMed]
- Hauri D. Can gastric pouch as orthotopic bladder replacement be used in adults? J Urol 1996;156:931-5. [Crossref] [PubMed]
- Lin DW, Santucci RA, Mayo ME, et al. Urodynamic evaluation and long-term results of the orthotopic gastric neobladder in men. J Urol 2000;164:356-9. [Crossref] [PubMed]
- Castellan M, Gosalbez R, Bar-Yosef Y, et al. Complications after use of gastric segments for lower urinary tract reconstruction. J Urol 2012;187:1823-7. [Crossref] [PubMed]
- Golimbu M, Morales P. Jejunal conduits: technique and complications. J Urol 1975;113:787-95. [Crossref] [PubMed]
- Clark SS. Electrolyte disturbance associated with jejunal conduit. J Urol 1974;112:42-7. [Crossref] [PubMed]
- Fontaine E, Barthelemy Y, Houlgatte A, et al. Twenty-year experience with jejunal conduits. Urology 1997;50:207-13. [Crossref] [PubMed]
- Casanova GA, Springer JP, Gerber E, et al. Urodynamic and clinical aspects of ileal low pressure bladder substitutes. Br J Urol 1993;72:728-35. [Crossref] [PubMed]
- Studer UE, Zingg EJ. Ileal orthotopic bladder substitutes. What we have learned from 12 years' experience with 200 patients. Urol Clin North Am 1997;24:781-93. [Crossref] [PubMed]
- Studer UE, Danuser H, Merz VW, et al. Experience in 100 patients with an ileal low pressure bladder substitute combined with an afferent tubular isoperistaltic segment. J Urol 1995;154:49-56. [Crossref] [PubMed]
- Hautmann RE, Miller K, Steiner U, et al. The ileal neobladder: 6 years of experience with more than 200 patients. J Urol 1993;150:40-5. [Crossref] [PubMed]
- Mills RD, Studer UE. Metabolic consequences of continent urinary diversion. J Urol 1999;161:1057-66. [Crossref] [PubMed]
- Pfitzenmaier J, Lotz J, Faldum A, et al. Metabolic evaluation of 94 patients 5 to 16 years after ileocecal pouch (Mainz pouch 1) continent urinary diversion. J Urol 2003;170:1884-7. [Crossref] [PubMed]
- Bastian PJ, Albers P, Hanitzsch H, et al. Health-related quality-of-life following modified ureterosigmoidostomy (Mainz Pouch II) as continent urinary diversion. Eur Urol 2004;46:591-7. [Crossref] [PubMed]
- Schmidt JD, Hawtrey CE, Buchsbaum HJ. Transverse colon conduit: a preferred method of urinary diversion for radiation-treated pelvic malignancies. J Urol 1975;113:308-13. [Crossref] [PubMed]
- Scardino PT, Bagley DH, Javadpour N, et al. Sigmoid conduit urinary diversion. Urology 1975;6:167-71. [Crossref] [PubMed]
- Richie JP, Skinner DG. Urinary diversion: the physiological rationale for non-refluxing colonic conduits. Br J Urol 1975;47:269-75. [Crossref] [PubMed]
- Kristjánsson A, Bajc M, Wallin L, et al. Renal function up to 16 years after conduit (refluxing or anti-reflux anastomosis) or continent urinary diversion. 2. Renal scarring and location of bacteriuria. Br J Urol 1995;76:546-50. [Crossref] [PubMed]
- Kristjánsson A, Wallin L, Mansson W. Renal function up to 16 years after conduit (refluxing or anti-reflux anastomosis) or continent urinary diversion. 1. Glomerular filtration rate and patency of uretero-intestinal anastomosis. Br J Urol 1995;76:539-45. [Crossref] [PubMed]
- Shapiro SR, Lebowitz R, Colodny AH. Fate of 90 children with ileal conduit urinary diversion a decade later: analysis of complications, pyelography, renal function and bacteriology. J Urol 1975;114:289-95. [Crossref] [PubMed]
- Roth S, van Ahlen H, Semjonow A, et al. Does the success of ureterointestinal implantation in orthotopic bladder substitution depend more on surgeon level of experience or choice of technique? J Urol 1997;157:56-60. [Crossref] [PubMed]
- Pantuck AJ, Han KR, Perrotti M, et al. Ureteroenteric anastomosis in continent urinary diversion: long-term results and complications of direct versus nonrefluxing techniques. J Urol 2000;163:450-5. [Crossref] [PubMed]
- Shaaban AA, Abdel-Latif M, Mosbah A, et al. A randomized study comparing an antireflux system with a direct ureteric anastomosis in patients with orthotopic ileal neobladders. BJU Int 2006;97:1057-62. [Crossref] [PubMed]
- Shigemura K, Yamanaka N, Imanishi O, et al. Wallace direct versus anti-reflux Le Duc ureteroileal anastomosis: comparative analysis in modified Studer orthotopic neobladder reconstruction. Int J Urol 2012;19:49-53. [Crossref] [PubMed]
- Hautmann RE. Urinary diversion: ileal conduit to neobladder. J Urol 2003;169:834-42. [Crossref] [PubMed]
- Hautmann RE, Volkmer BG, Schumacher MC, et al. Long-term results of standard procedures in urology: the ileal neobladder. World J Urol 2006;24:305-14. [Crossref] [PubMed]
- Regan JB, Barrett DM. Stented versus nonstented ureteroileal anastomoses: is there a difference with regard to leak and stricture? J Urol 1985;134:1101-3. [Crossref] [PubMed]
- Bricker EM. Bladder substitution after pelvic evisceration. Surg Clin North Am 1950;30:1511-21. [Crossref] [PubMed]
- Nesbit RM. Ureterosigmoid anastomosis by direct elliptical connection; a preliminary report. J Urol 1949;61:728-34. [Crossref] [PubMed]
- Wallace DM. Uretero-ileostomy. Br J Urol 1970;42:529-34. [Crossref] [PubMed]
- Davis NF, Burke JP, McDermott T, et al. Bricker versus Wallace anastomosis: A meta-analysis of ureteroenteric stricture rates after ileal conduit urinary diversion. Can Urol Assoc J 2015;9:E284-90. [Crossref] [PubMed]
- Evangelidis A, Lee EK, Karellas ME, et al. Evaluation of ureterointestinal anastomosis: Wallace vs Bricker. J Urol 2006;175:1755-8; discussion 1758. [Crossref] [PubMed]
- Coffey RC. Transplantation of Ureters Into Large Intestine by Submucous Implantation. JAMA 1932;99:1320-3. [Crossref]
- Leadbetter WF, Clarke BG. Five years' experience with uretero-enterostomy by the combined technique. J Urol 1955;73:67-82. [Crossref] [PubMed]
- Goodwin WE, Harris AP, Kaufman JJ, et al. Open, transcolonic ureterointestinal anastomosis; a new approach. Surg Gynecol Obstet 1953;97:295-300. [PubMed]
- Strickler WL. A Modification of the Combined Ureterosigmoidostomy. J Urol 1965;93:370-3. [Crossref] [PubMed]
- Pagano F. Ureterocolonic anastomosis: description of a technique. J Urol 1980;123:355-6. [Crossref] [PubMed]
- Starr A, Rose DH, Cooper F. Antireflux ureteroileal anastomoses in humans. J Urol 1975;113:170-4. [Crossref] [PubMed]
- Warwick RT, Ashken MH. The functional results of partial, subtotal and total cystoplasty with special reference to ureterocaecocystoplasty, selective sphincterotomy and cystocystoplasty. Br J Urol 1967;39:3-12. [Crossref] [PubMed]
- Le Duc A, Camey M, Teillac P. An original antireflux ureteroileal implantation technique: long-term followup. J Urol 1987;137:1156-8. [Crossref] [PubMed]
- Wishahi MM, Elganzoury H, Elkhouly A, et al. Dipping technique for ureteroileal anastomosis in orthotopic ileal neobladder: 20-year experience in 670 patients-no stenosis with preservation of the upper tract. ISRN Urol 2013;2013:725286. [Crossref] [PubMed]
- Hautmann RE, Abol-Enein H, Lee CT, et al. Urinary diversion: how experts divert. Urology 2015;85:233-8. [Crossref] [PubMed]
- Gore JL, Litwin MS. Urologic Diseases in America P. Quality of care in bladder cancer: trends in urinary diversion following radical cystectomy. World J Urol 2009;27:45-50. [Crossref] [PubMed]
- Johnson DE, Lamy SM. Complications of a single stage radical cystectomy and ileal conduit diversion: review of 214 cases. J Urol 1977;117:171-3. [Crossref] [PubMed]
- Madersbacher S, Schmidt J, Eberle JM, et al. Long-term outcome of ileal conduit diversion. J Urol 2003;169:985-90. [Crossref] [PubMed]
- Donahue TF, Bochner BH. Parastomal hernias after radical cystectomy and ileal conduit diversion. Investig Clin Urol 2016;57:240-8. [Crossref] [PubMed]
- Donahue TF, Cha EK, Bochner BH. Rationale and Early Experience with Prophylactic Placement of Mesh to Prevent Parastomal Hernia Formation after Ileal Conduit Urinary Diversion and Cystectomy for Bladder Cancer. Curr Urol Rep 2016;17:9. [Crossref] [PubMed]
- Dutta SC, Chang SC, Coffey CS, et al. Health related quality of life assessment after radical cystectomy: comparison of ileal conduit with continent orthotopic neobladder. J Urol 2002;168:164-7. [Crossref] [PubMed]
- Ali AS, Hayes MC, Birch B, et al. Health related quality of life (HRQoL) after cystectomy: comparison between orthotopic neobladder and ileal conduit diversion. Eur J Surg Oncol 2015;41:295-9. [Crossref] [PubMed]
- Hart S, Skinner EC, Meyerowitz BE, et al. Quality of life after radical cystectomy for bladder cancer in patients with an ileal conduit, cutaneous or urethral kock pouch. J Urol 1999;162:77-81. [Crossref] [PubMed]
- Lilien OM, Camey M. 25-year experience with replacement of the human bladder (Camey procedure). J Urol 1984;132:886-91. [Crossref] [PubMed]
- Studer UE, Ackermann D, Casanova GA, et al. Three years' experience with an ileal low pressure bladder substitute. Br J Urol 1989;63:43-52. [Crossref] [PubMed]
- Hautmann RE, Egghart G, Frohneberg D, et al. The ileal neobladder. J Urol 1988;139:39-42. [Crossref] [PubMed]
- Thüroff JW, Alken P, Riedmiller H, et al. The Mainz pouch (mixed augmentation ileum and cecum) for bladder augmentation and continent diversion. J Urol 1986;136:17-26. [Crossref] [PubMed]
- Light JK, Engelmann UH. Le bag: total replacement of the bladder using an ileocolonic pouch. J Urol 1986;136:27-31. [Crossref] [PubMed]
- Reddy PK, Lange PH. Bladder replacement with sigmoid colon after radical cystoprostatectomy. Urology 1987;29:368-71. [Crossref] [PubMed]
- Hautmann RE, de Petriconi RC, Volkmer BG. 25 years of experience with 1,000 neobladders: long-term complications. J Urol 2011;185:2207-12. [Crossref] [PubMed]
- Ghoneim MA, Shehab-El-Din AB, Ashamallah AK, et al. Evolution of the rectal bladder as a method for urinary diversion. J Urol 1981;126:737-40. [Crossref] [PubMed]
- Kock NG, Ghoneim MA, Lycke KG, et al. Urinary diversion to the augmented and valved rectum: preliminary results with a novel surgical procedure. J Urol 1988;140:1375-9. [Crossref] [PubMed]
- Fisch M, Wammack R, Hohenfellner R. The sigma rectum pouch (Mainz pouch II). World J Urol 1996;14:68-72. [Crossref] [PubMed]
- Bracci U. Urinary diversion by the Heitz-Boyer-Hovelacque procedure. Technique and experience. Urol Int 1968;23:63-73. [Crossref] [PubMed]
- Leadbetter GW Jr, Zickerman P, Pierce E. Ureterosigmoidostomy and carcinoma of the colon. J Urol 1979;121:732-5. [Crossref] [PubMed]
- Ferris DO, Odel HM. Electrolyte pattern of the blood after bilateral ureterosigmoidostomy. J Am Med Assoc 1950;142:634-41. [Crossref] [PubMed]
- Gilchrist RK, Merricks JW, Hamlin HH, et al. Construction of a substitute bladder and urethra. Surg Gynecol Obstet 1950;90:752-60. [PubMed]
- Rowland RG, Mitchell ME, Bihrle R, et al. Indiana continent urinary reservoir. J Urol 1987;137:1136-9. [Crossref] [PubMed]
- Skinner DG, Lieskovsky G, Boyd SD. Technique of creation of a continent internal ileal reservoir (Kock pouch) for urinary diversion. Urol Clin North Am 1984;11:741-9. [PubMed]
- Stein JP, Skinner DG. T-mechanism applied to urinary diversion: the orthotopic T-pouch ileal neobladder and cutaneous double-T-pouch ileal reservoir. Tech Urol 2001;7:209-22. [PubMed]
- Thüroff JW, Alken P, Riedmiller H, et al. 100 cases of Mainz pouch: continuing experience and evolution. J Urol 1988;140:283-8. [Crossref] [PubMed]
- Duckett JW, Snyder HM 3rd. Continent urinary diversion: variations on the Mitrofanoff principle. J Urol 1986;136:58-62. [Crossref] [PubMed]
- b1p4el A, Thüroff JW. Bowel-flap tubes for continent cutaneous urinary diversion. World J Urol 1998;16:235-41. [Crossref] [PubMed]
- Holmes DG, Thrasher JB, Park GY, et al. Long-term complications related to the modified Indiana pouch. Urology 2002;60:603-6. [Crossref] [PubMed]
- Gill IS, Fergany A, Klein EA, et al. Laparoscopic radical cystoprostatectomy with ileal conduit performed completely intracorporeally: the initial 2 cases. Urology 2000;56:26-9; discussion 269-30. [Crossref] [PubMed]
- Shi H, Li J, Li K, et al. Minimally invasive versus open radical cystectomy for bladder cancer: A systematic review and meta-analysis. J Int Med Res 2019;47:4604-18. [Crossref] [PubMed]
- Albisinni S, Veccia A, Aoun F, et al. A systematic review and meta-analysis comparing the outcomes of open and robotic assisted radical cystectomy. Minerva Urol Nefrol 2019;71:553-68. [Crossref] [PubMed]
- Palazzetti A, Sanchez-Salas R, Capogrosso P, et al. Systematic review of perioperative outcomes and complications after open, laparoscopic and robot-assisted radical cystectomy. Actas Urol Esp 2017;41:416-25. [Crossref] [PubMed]
- Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet 2018;391:2525-36. [Crossref] [PubMed]
- Venkatramani V, Reis IM, Castle EP, et al. Predictors of Recurrence, and Progression-Free and Overall Survival following Open versus Robotic Radical Cystectomy: Analysis from the RAZOR Trial with a 3-Year Followup. J Urol 2020;203:522-9. [Crossref] [PubMed]
- Pruthi RS, Nix J, McRackan D, et al. Robotic-assisted laparoscopic intracorporeal urinary diversion. Eur Urol 2010;57:1013-21. [Crossref] [PubMed]
- Ahmed K, Khan SA, Hayn MH, et al. Analysis of intracorporeal compared with extracorporeal urinary diversion after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol 2014;65:340-7. [Crossref] [PubMed]
- Lenfant L, Verhoest G, Campi R, et al. Perioperative outcomes and complications of intracorporeal vs extracorporeal urinary diversion after robot-assisted radical cystectomy for bladder cancer: a real-life, multi-institutional french study. World J Urol 2018;36:1711-8. [Crossref] [PubMed]
- Carrion A, Pinero A, Raventos C, et al. Comparison of perioperative outcomes and complications of robot assisted radical cystectomy with extracorporeal vs intracorporeal urinary diversion. Actas Urol Esp 2019;43:277-83. [Crossref] [PubMed]
- Zhang JH, Ericson KJ, Thomas LJ, et al. Large Single Institution Comparison of Perioperative Outcomes and Complications of Open Radical Cystectomy, Intracorporeal Robot-Assisted Radical Cystectomy and Robotic Extracorporeal Approach. J Urol 2020;203:512-21. [Crossref] [PubMed]
- Hayn M, Stegemann A, Consiglio J, et al. Comparison of complications and validated quality of life outcomes between intra-corporeal and extra-corporeal ileal conduit after robot-assisted radical cystectomy. J Urol 2011;185:e459. [Crossref]
- Bertolo R, Agudelo J, Garisto J, et al. Perioperative Outcomes and Complications after Robotic Radical Cystectomy With Intracorporeal or Extracorporeal Ileal Conduit Urinary Diversion: Head-to-head Comparison From a Single-Institutional Prospective Study. Urology 2019;129:98-105. [Crossref] [PubMed]
- Tan TW, Nair R, Saad S, et al. Safe transition from extracorporeal to intracorporeal urinary diversion following robot-assisted cystectomy: a recipe for reducing operative time, blood loss and complication rates. World J Urol 2019;37:367-72. [Crossref] [PubMed]
- Kaouk J, Garisto J, Eltemamy M, et al. Single-port Robotic Intracorporeal Ileal Conduit Urinary Diversion During Radical Cystectomy Using the SP Surgical System: Step-by-step Technique. Urology 2019;130:196-200. [Crossref] [PubMed]
- Garisto J, Bertolo R, Kaouk J. Transperineal Approach for Intracorporeal Ileal Conduit Urinary Diversion Using a Purpose-built Single-port Robotic System: Step-by-step. Urology 2018;122:179-84. [Crossref] [PubMed]
- Haber GP, Campbell SC, Colombo JR Jr, et al. Perioperative outcomes with laparoscopic radical cystectomy: "pure laparoscopic" and "open-assisted laparoscopic" approaches. Urology 2007;70:910-5. [Crossref] [PubMed]
- Kanno T, Kobori G, Shibasaki N, et al. Laparoscopic intracorporeal ileal conduit after laparoscopic radical cystectomy: A modified technique to facilitate ureteroenteric anastomosis. Int J Urol 2018;25:976-8. [Crossref] [PubMed]
- Wu L, Yang F, Song L, et al. Comparison of intracorporeal and extracorporeal urinary diversions after laparoscopic radical cystectomy in females with bladder cancer. World J Surg Oncol 2019;17:161. [Crossref] [PubMed]
- Perlin DV, Alexandrov IV, Zippunnikov VP, et al. Urologiia 2019;54-9. [Laparoscopic radical cystectomy: novel technique with late dividing of the ureters]. [PubMed]
- Wang MS, He QB, Yang FY, et al. A Retrospective Study Comparing Surgical and Early Oncological Outcomes between Intracorporeal and Extracorporeal Ileal Conduit after Laparoscopic Radical Cystectomy from a Single Center. Chin Med J (Engl) 2018;131:784-9. [Crossref] [PubMed]
- Beecken WD, Wolfram M, Engl T, et al. Robotic-assisted laparoscopic radical cystectomy and intra-abdominal formation of an orthotopic ileal neobladder. Eur Urol 2003;44:337-9. [Crossref] [PubMed]
- Dason S, Goh AC. Contemporary techniques and outcomes of robotic cystectomy and intracorporeal urinary diversions. Curr Opin Urol 2018;28:115-22. [Crossref] [PubMed]
- Goh AC, Aghazadeh MA, Krasnow RE, et al. Robotic Intracorporeal Continent Cutaneous Urinary Diversion: Primary Description. J Endourol 2015;29:1217-20. [Crossref] [PubMed]
- Desai MM, Simone G, de Castro Abreu AL, et al. Robotic Intracorporeal Continent Cutaneous Diversion. J Urol 2017;198:436-44. [Crossref] [PubMed]
- Matulewicz RS, Chesnut GT, Huang CC, et al. Evolution in technique of robotic intracorporeal continent catheterizable pouch after cystectomy. Urol Video J 2019;4:100020. [Crossref] [PubMed]
- Atala A, Bauer SB, Soker S, et al. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006;367:1241-6. [Crossref] [PubMed]
- Shakhssalim N, Soleimani M, Dehghan MM, et al. Bladder smooth muscle cells on electrospun poly(epsilon-caprolactone)/poly(l-lactic acid) scaffold promote bladder regeneration in a canine model. Mater Sci Eng C Mater Biol Appl 2017;75:877-84. [Crossref] [PubMed]
- Zhao Y, He Y, Guo JH, et al. Time-dependent bladder tissue regeneration using bilayer bladder acellular matrix graft-silk fibroin scaffolds in a rat bladder augmentation model. Acta Biomater 2015;23:91-102. [Crossref] [PubMed]
- Wang Y, Zhou S, Yang R, et al. Bioengineered bladder patches constructed from multilayered adipose-derived stem cell sheets for bladder regeneration. Acta Biomater 2019;85:131-41. [Crossref] [PubMed]
- Young DA, McGilvray KC, Ehrhart N, et al. Comparison of in vivo remodeling of urinary bladder matrix and acellular dermal matrix in an ovine model. Regen Med 2018;13:759-73. [Crossref] [PubMed]
- Shi C, Chen W, Chen B, et al. Bladder regeneration in a canine model using a bladder acellular matrix loaded with a collagen-binding bFGF. Biomater Sci 2017;5:2427-36. [Crossref] [PubMed]
- Sopko NA, Kates M, Bivalacqua TJ. Use of regenerative tissue for urinary diversion. Curr Opin Urol 2015;25:578-85. [Crossref] [PubMed]
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997;78:606-17. [Crossref] [PubMed]
- Mukhtar S, Ayres BE, Issa R, et al. Challenging boundaries: an enhanced recovery programme for radical cystectomy. Ann R Coll Surg Engl 2013;95:200-6. [Crossref] [PubMed]
- Dunkman WJ, Manning MW, Whittle J, et al. Impact of an enhanced recovery pathway on length of stay and complications in elective radical cystectomy: a before and after cohort study. Perioper Med (Lond) 2019;8:9. [Crossref] [PubMed]
- Pang KH, Groves R, Venugopal S, et al. Prospective Implementation of Enhanced Recovery After Surgery Protocols to Radical Cystectomy. Eur Urol 2018;73:363-71. [Crossref] [PubMed]
- Zhang H, Wang H, Zhu M, et al. Implementation of enhanced recovery after surgery in patients undergoing radical cystectomy: A retrospective cohort study. Eur J Surg Oncol 2020;46:202-8. [Crossref] [PubMed]
- Liu B, Domes T, Jana K. Evaluation of an enhanced recovery protocol on patients having radical cystectomy for bladder cancer. Can Urol Assoc J 2018;12:421-6. [Crossref] [PubMed]
- Wei C, Wan F, Zhao H, et al. Application of enhanced recovery after surgery in patients undergoing radical cystectomy. J Int Med Res 2018;46:5011-8. [Crossref] [PubMed]
- Semerjian A, Milbar N, Kates M, et al. Hospital Charges and Length of Stay Following Radical Cystectomy in the Enhanced Recovery After Surgery Era. Urology 2018;111:86-91. [Crossref] [PubMed]
- Tan WS, Tan MY, Lamb BW, et al. Intracorporeal robot-assisted radical cystectomy, together with an enhanced recovery programme, improves postoperative outcomes by aggregating marginal gains. BJU Int 2018;121:632-9. [Crossref] [PubMed]
- Pogatschnik C, Steiger E. Review of Preoperative Carbohydrate Loading. Nutr Clin Pract 2015;30:660-4. [Crossref] [PubMed]
- Winer AG, Sfakianos JP, Puttanniah VG, et al. Comparison of perioperative outcomes for epidural versus intravenous patient-controlled analgesia after radical cystectomy. Reg Anesth Pain Med 2015;40:239-44. [Crossref] [PubMed]
- Metcalf M, Glazyrine V, Glavin K, et al. The Feasibility of a Health Care Application in the Treatment of Patients Undergoing Radical Cystectomy. J Urol 2019;201:902-8. [Crossref] [PubMed]
Cite this article as: Igel DA, Chestnut CJ, Lee EK. Urinary diversion and reconstruction following radical cystectomy for bladder cancer: a narrative review. AME Med J 2021;6:4.

