
High-pressure continuous suction drainage for thoracic empyema with pulmonary fistula
Introduction
The treatment of thoracic empyema involves administration of antibiotics and efficient drainage of pus from the thorax in order to control the infection. Open window thoracostomy (OWT) should be offered for efficient drainage, especially for thoracic empyema with pulmonary fistula (PF), which continuously supplies infected pus into the thoracic cavity. Although conventional OWT offers excellent drainage, postoperative quality of life may be impaired, owing to postoperative pain associated with rib resection, and it necessitates subsequent surgery to close the chest wall.
The method of treatment of thoracic empyema has been changing. The first reports on intrapleural vacuum-assisted closure (VAC) therapy were published in 2006 (1). Later reports (2-5) demonstrated that VAC therapy can accelerate the treatment of complex thoracic empyema after OWT. Hofmann [2012] et al. (6) reported a case that was successfully treated by VAC therapy without a preceding thoracostomy. However, VAC therapy requires proficient staff and careful management, such as changing of sponges every two to three days. VAC therapy method cannot be offered in every institution. A simpler and commoner method should be considered.
We described a consecutive case series of thoracic empyema with PF that was successfully treated with high-pressure continuous suction drainage (HCSD) alone. To our knowledge, this was the first article to outline multiple cases of thoracic empyema with PF that were treated without either OWT or VAC devices.
Methods
We retrospectively investigated six consecutive cases of thoracic empyema with PF between January 1, 2015 and December 31, 2018. The diagnosis of thoracic empyema was made by laboratory, radiologic, and microbiologic examinations. PF was confirmed by thin-slice computed tomography (CT). We aimed to discharge patients without any unfilled pleural cavities. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Akashi Medical Center institutional review board (2020-30), and written informed consent was obtained from each patient.
Surgical procedure
Preparatory surgery was generally performed to decrease the number of bacteria and fungi and to properly place drainage tubes. Video-assisted thoracic surgery was adopted for preparatory debridement and decortication. The thoracic cavity was irrigated with saline solution, followed by placement of one or two 20–24-Fr. thoracic drainage tubes, depending on the intrathoracic conditions.
HCSD procedure
Drainage tubes were connected to a continuous suction unit (MERA Sucuum; MERA, Tokyo, Japan) (Figure 1). Suction was initially set at −20 cmH2O and was increased incrementally up to −50 cmH2O, which was the maximum set pressure of the suction units. Utmost care was taken to identify any possible changes in the patients’ circulatory and respiratory dynamics when the set pressure was changed. Intrathoracic pressure and the presence of air leakage were evaluated based on the number displayed on the unit. The drainage tube was removed when the pleural cavity was completely obliterated with granulation tissue and no air leakage was detected. When the drainage was deemed insufficient and the intrathoracic infection was out of control, conversion to OWT was considered without hesitation. The patients with PF treated for lung cancer were to be followed up for five years as our conventional manner. The patients without lung cancer were followed for roughly one year after discharge to see if there were any changes inside thoracic cavity.

Corresponding nursing care
The drain insertion site should be observed regularly. As the duration of drain placement is relatively long, there is some possibility that the drain tube can fall off easily due to enlargement of the drain inserting stoma.
Effect measurement
Pleural fluid cultures were taken roughly on a weekly basis, with appropriate antibiotics added accordingly. Blood samples and X-rays were taken regularly to see if the management was effective. CT scans were used for detailed evaluation of the thoracic cavity.
Antibiotic administration
Appropriate antibiotics that were targeted to the isolated bacteria or fungi were administered and continued for 2–4 weeks, even after drainage tube removal, depending on the condition of the patients.
Results
Course and outcome of continuous suction drainage
The patient demographics are summarized in Table 1. All patients were men, with a mean age of 65.0 years (range, 61–69 years). The causes of thoracic empyema with PF were intraoperative parenchymal injury in four patients and pneumonia in two patients. Four patients required surgical intervention for the primary disease (cases 3–6), and three patients were taking medications for diabetes mellitus. All patients, except case 3 (patient’s refusal), underwent preparatory surgical debridement before the HCSD treatment. Various bacteria and fungi were isolated from the pleural fluid culture and were as follows: Pseudomonas species, Staphylococcus species, Enterobacter species, Candida tropicalis, and Corynebacterium species.
Table 1
| Patient | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| Age (year) | 61 | 61 | 69 | 64 | 66 | 69 |
| Sex | Male | Male | Male | Male | Male | Male |
| Diagnosis | Pneumonia | Pneumonia | NSCLC | NSCLC | NTM | Meso. |
| Cause | Pneumonia | Pneumonia | intra-OP | intra-OP | intra-OP | intra-OP |
| PYI | 20 | 43 | 37 | 80 | 0 | 47 |
| Diabetes | + | − | + | + | − | − |
| Laterality | Left | Left | Left | Right | Left | Right |
| Debridement | Done | Done | Undone | Done | Done | Done |
| PFC | Staph. | Ent. | Pseudo. | Ent. | Pseudo. | Candida |
+, presence; −, absence. Ent., Enterobacter species; intra-OP, intra-operative injury; Meso., pleural mesothelioma, NSCLC, non-small cell lung carcinoma; NTM, non-tuberculous mycobacterial infection; PFC, pleural fluid culture; Pseudo., Pseudomonas species; PYI, pack-year index; Staph., Staphylococcus species.
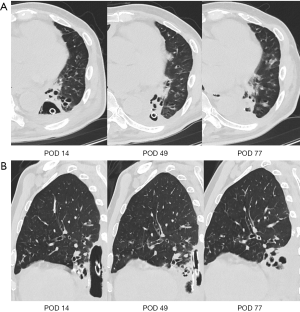
The outcomes of HCSD treatment are shown in Table 2. All six patients were successfully treated by HCSD alone. The patients tolerated a suction of –50 cmH2O without any associated complications, including arrhythmia or mediastinal shift. None required conversion to conventional OWT. The mean period of HCSD treatment was 60.2 days (range, 27–105 days), and the mean duration of air leakage was 57.2 days (range, 22–100 days). To accelerate the treatment, endobronchial Watanabe spigot (EWS) was placed in three patients. In three cases (cases 3, 4, and 6) that initially had some bacterial colonies, the bacteria completely disappeared by the time of tube removal. In all cases, the dimension of the pleural cavity was decreased and filled with granulation tissue (Figure 2A,B). None of our patients developed associated complications and recurrence of empyema within a median follow-up period of 643.0 days (range, 216–1,245 days).
Table 2
| Patient | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| Age (y. o.) | 61 | 61 | 69 | 64 | 66 | 69 |
| Sex | Male | Male | Male | Male | Male | Male |
| Disease | Pneumonia | Pneumonia | NSCLC | NSCLC | NTM | Meso. |
| Cause | Pneumonia | Pneumonia | Intra-OP | Intra-OP | Intra-OP | Intra-OP |
| EWS | Undone | Undone | Done | Done | Undone | Done |
| AL stops (POD) | 55 | 43 | 50 | 22 | 73 | 100 |
| Drain tube removal (POD) | 56 | 45 | 52 | 27 | 76 | 105 |
| PFC | Staph. | No growth | No Growth | Ent. | Pseudo. | No growth |
| Complications | None (respectively) | None (respectively) | None (respectively) | None (respectively) | None (respectively) | None (respectively) |
| Conversion to OWT | None (respectively) | None (respectively) | None (respectively) | None (respectively) | None (respectively) | None (respectively) |
| Follow-up (day) | 1,245 | 1,008 | 588 | 445 | 356 | 216 |
AL, air leakage; Ent., Enterobacter species; EWS, endobronchial Watanabe spigot; intra-OP, intra-operative injury; Meso., pleural mesothelioma; NSCLC, non-small cell lung carcinoma; NTM, non-tuberculous mycobacterial infection; OWT, open-window thoracostomy; PFC, pleural fluid culture; POD, postoperative day; Pseudo., Pseudomonas species; Staph., Staphylococcus species.
Discussion
Thoracic empyema with PF is refractory and challenging to treat. In cases that require long-term administration of antibiotics and adequate drainage, OWT can facilitate excellent drainage of collected pus (7). However, despite widespread acceptance, OWT has disadvantages (4), including postoperative pain associated with rib resection, unaesthetic appearance of a cavity on the chest, and the necessity for a subsequent operation for chest wall closure. Hato [2014] et al. (8) reported that OWT closure was achieved in only 34.3% of patients. Furthermore, Palmen [2009] et al. (3) reported that half of their patients died of OWT-related complications, such as bleeding and recurrent infections, during follow-up. The common causes of death after OWT are sepsis and multiorgan failure (3,8-10).
Several recent reports (1-5) have demonstrated the efficacy of VAC therapy for patients treated with conventional OWT. Although this method shortens the treatment period, OWT is needed in advance. Some reports (6,11,12) presented cases that were successfully treated by VAC therapy without a preceding OWT. Nevertheless, VAC therapy requires experienced staff and careful management, such as changing of sponges every two to three days. In this present report, we described consecutive cases of thoracic empyema with PF that were successfully treated with continuous suction drainage alone.
HCSD
There are some potential benefits in HCSD. Firstly, only the readily available devices are used, which do not require skilled specialists for operation. Secondly, continuous drainage can provide a relatively clean environment by keeping the number of bacteria and fungi low. In our institution, suction pressure is typically set at −15 cmH2O for patients with thoracic empyema. In the present study, suction pressure was eventually increased up to −50 cmH2O, which may have allowed more efficient drainage of intrathoracic pus. We hypothesized that negative pressure would enhance expansion of the residual lung and advance the proliferation of granulation tissue, similar to the effects of VAC treatment. During VAC therapy, a maximum pressure of −125 mmHg is applied to the chest cavity; by far, there had been no reports on ipsilateral mediastinal shift or any other associated complications with this technique (1-6,11,12). In this present study, no cases needed discontinuation of the HCSD or conversion to OWT because of associated complications. Although we considered the application of more negative pressure, −50 cmH2O was the maximum set pressure of the suction unit that we used. Further studies should try other units that have even higher suction pressure.
Thirdly, OWT can be avoided which causes several complications as mentioned above.
Lastly, HCSD method is conservative and minimally invasive, and we believe patients with other underlying diseases who may not be suitable for surgical intervention can tolerate this method. At least, all the patients in our study completed this therapy.
Timing of drainage tube removal
The drainage tube was removed when the pleural cavity was completely filled with granulation tissue and no air leakage was detected. The presence of remaining infection on the latest culture result was not a factor for the decision on drainage tube removal, because we assumed that the tube can be safely removed when there was no more space for bacteria or fungi to proliferate. If drain tube is removed too early, recurrence of thoracic empyema will be likely as there still remains some space for air leaks from lung to come out.
In our study, drainage tubes were removed on postoperative day 60.2 on the average (range, postoperative day 27–105). In three patients, EWS was placed to accelerate treatment and was effective in decreasing air leakage. Therefore, EWS should be considered for patients with major air leakage.
Duration of therapy
The duration of OWT is relatively long. Palmen [2009] (3) reported that the OWT was created 58±119 days after the diagnosis of the empyema and that the length of hospital stay after the OWT was 60±41 days with additional VAC treatment. Patients later underwent surgery for closure. In another report (2), OWT was created 52 days (range, 21–126 days) after the primary intervention, with a mean hospital stay of 22.7 days after OWT and VAC installation. Closure of the OWT was planned after a mean period of three months. In both reports, OWT was delayed after the diagnosis of empyema. In our study, the mean period of HCSD treatment was 60.2 days (range, 27–105 days), and there was no need for subsequent surgery to close the chest wall. EWS may shorten the duration of HCSD.
Study limitations
This study had some limitations, such as the retrospective observational design and the small number of patients. Future large-scale prospective study will be required to conclude if HCSD is effective for thoracic empyema with PF.
Conclusions
HCSD treatment was safe, minimally invasive, and effective for patients with thoracic empyema with PF and may be considered as an alternative treatment to OWT.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at https://amj.amegroups.com/article/view/10.21037/amj-20-151/dss
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-20-151/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-151/coif). YT serves as an unpaid editorial board member of AME Medical Journal from August 2019 to August 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethic board of Akashi Medical Center (2020-30) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Varker KA, Ng T. Management of empyema cavity with the vacuum-assisted closure device. Ann Thorac Surg 2006;81:723-5. [Crossref] [PubMed]
- Sziklavari Z, Grosser C, Neu R, et al. Complex pleural empyema can be safely treated with vacuum-assisted closure. J Cardiothorac Surg 2011;6:130. [Crossref] [PubMed]
- Palmen M, van Breugel HN, Geskes GG, et al. Open window thoracostomy treatment of empyema is accelerated by vacuum-assisted closure. Ann Thorac Surg 2009;88:1131-6. [Crossref] [PubMed]
- Sziklavari Z, Ried M, Zeman F, et al. Short-term and long-term outcomes of intrathoracic vacuum therapy of empyema in debilitated patients. J Cardiothorac Surg 2016;11:148. [Crossref] [PubMed]
- Saadi A, Perentes JY, Gonzalez M, et al. Vacuum-assisted closure device: a useful tool in the management of severe intrathoracic infections. Ann Thorac Surg 2011;91:1582-9. [Crossref] [PubMed]
- Hofmann HS, Schemm R, Grosser C, et al. Vacuum-assisted closure of pleural empyema without classic open-window thoracostomy. Ann Thorac Surg 2012;93:1741-2. [Crossref] [PubMed]
- Molnar TF. Current surgical treatment of thoracic empyema in adults. Eur J Cardiothorac Surg 2007;32:422-30. [Crossref] [PubMed]
- Hato T, Suzuki S, Harada M, et al. Comprehensive treatment approach is necessary for the closure of open window thoracostomy: an institutional review of 35 cases. Surg Today 2014;44:443-8. [Crossref] [PubMed]
- Mazzella A, Pardolesi A, Maisonneuve P, et al. Bronchopleural Fistula After Pneumonectomy: Risk Factors and Management, Focusing on Open-Window Thoracostomy. Semin Thorac Cardiovasc Surg 2018;30:104-13. [Crossref] [PubMed]
- Segers P, Kloek JJ, Strackee SD, et al. Open window thoracostomy: a new therapeutic option using topical negative pressure wound therapy. Wounds 2007;19:264-9. [PubMed]
- Sziklavari Z, Ried M, Neu R, et al. Mini-open vacuum-assisted closure therapy with instillation for debilitated and septic patients with pleural empyema. Eur J Cardiothorac Surg 2015;48:e9-e16. [Crossref] [PubMed]
- Sziklavari Z, Grosser C, Neu R, et al. Minimally invasive vacuum-assisted closure therapy in the management of complex pleural empyema. Interact Cardiovasc Thorac Surg 2013;17:49-53. [Crossref] [PubMed]
Cite this article as: Uchida T, Tanaka Y, Tauchi S, Maniwa Y. High-pressure continuous suction drainage for thoracic empyema with pulmonary fistula. AME Med J 2021;6:13.

