
Surgical principles of penile cancer for penectomy and inguinal lymph node dissection: a narrative review
Introduction
Penile cancer is a rare and serious disease with an estimated 2,080 new cases and 410 deaths reported in the United States in 2019. Major risk factors include infectious (HPV, HIV) and inflammatory (smoking, poor hygiene, lichen sclerosis and balanitis) conditions, as well as lower socio-economic status. While penile cancer is rare in the developed world, with rates as low as 0.3–0.6 per 100,000 in the United States and United Kingdom, penile cancer is less uncommon in developing nations, where rates as high as 2.8–6.8 per 100,000 have been reported (1,2). The rise in obesity and associated obesity-related acquired buried penis will likely increase penile cancer rates; as one study demonstrated a 53% increase in penile cancer incidence for every five-unit increase in body mass index (BMI) (2,3) (Figure 1). However, widespread HPV vaccination has the potential to lower penile cancer rates, though this has not yet been established in the literature (4).
Surgery forms the cornerstone of therapy for penile cancer. Early local and regional disease is surgically curable, but advanced regional disease portends a poor prognosis. The extent of inguinal node metastases is the most important prognostic factor with survival dropping sharply with increasing disease burden. The reported 5 year cancer-specific survival for pN3 is 0–17% compared to 17–60% for pN2, 79–89% for pN1 and 85–100% for pN0 (5,6). Prompt surgical intervention is key and a delay of 6 months can drastically reduce survival in patients with early microscopic lymph node disease (7). Unfortunately, it is well known that penile cancer presentation is delayed due to fear and stigma and this delay can be up to one year or even longer (8). Penile cancer patients may benefit from referral to academic centers, as patients at academic centers are significantly more likely to undergo guideline-based inguinal lymph node dissection (ILND) than community centers (48.4% vs. 26.6%) with higher node yield (18.5 vs. 12.5) (9). Principles of surgical management continue to evolve, with increased focus on minimizing morbidity without compromising oncologic safety. Here-in we provide a review on the current state of surgical care for penile cancer, both primary and inguinal nodal disease, selected from the published literature. We present the following article in accordance with the Narrative Review reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-20-159/rc).
Oncologically safe margin
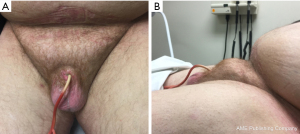
Partial penectomy and total penectomy remain the standard of care for penile cancer (Figures 2,3). Surgical techniques for these procedures have been previously well described (10,11). The decision to perform partial versus total penectomy depends the volume of disease, grade of the tumor, ability to obtain clear margins and body habitus. For patients with concealed penis, it is highly recommended to resect the surrounding tissue including the cicatrix and perform perineal urethrostomy (Figure 1). In our experience, these patients do not have enough phallic length to perform a partial penectomy.
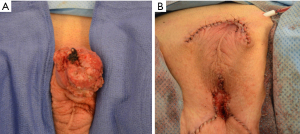
Originally, surgical resection with at least a 2 cm tumor-free margin was recommended to insure an oncologically safe margin and prevent local recurrence. In addition, the decision to proceed with a partial versus total penectomy depends on whether an adequate penile stump for functional upright urination could be preserved, typically 3–4 cm (Figure 3). Collectively, these clinical decision points led to more aggressive resections that cause considerable psychological distress and altered body image.
The 2 cm tumor-free margin was recommended arbitrarily and not based on histopathological evaluation or local recurrence. This recommendation was challenged by two landmark papers. First, Agrawal et al. evaluated serial 5 mm margins in 64 penectomy specimens for microscopic migration away from visible tumor and found that 81% of the tumors did not extend beyond the visible tumor margin and only 3 extended beyond 5 mm—all were high grade lesions. No skip lesions were identified in any of the specimens examined (12). A subsequent study by Minhas et al. evaluated surgical margins in 51 patients treated with penile sparing techniques—glansectomy, partial penectomy, and wide local excision. 92% of patients had <20 mm margin and 48% had <10 mm. There were 3 positive margins (3%) and 2 patients (4%) with high grade tumors developed tumor recurrence at 26 months (13). These papers concluded that a 2 cm tumor-free margin was unnecessary and less penile resection was required.
Subsequent studies have evaluated the safety of surgical margins on oncologic outcomes. Philippou et al. reviewed 179 patients undergoing conservative surgery for penile cancer. They found that the mean distance from the tumor edge to the skin margin was 5.23±5.78 mm and to the deep margin was 4.5±5.3 mm; 12 patients (6.7%) had a positive margin. Importantly, they identified that tumor grade, stage, and lymphovascular invasion were predictors of recurrence. Patients with isolated local recurrence had an overall 5-year disease specific survival of 91.7% (14). Similarly, Sri et al. reported that in 332 patients surgical margins of 5 mm did not increase the risk of local recurrence, but a margin of <1 mm, cavernosal invasion, and lymphovascular invasion significantly increased local recurrence risk (15). Collectively, these studies have influenced current guidelines to recommend 5 mm margins in lieu of the previous 2 cm recommendation (16). Although smaller margins are recommended, surgeons should use intraoperative frozen sections to guide the degree of resection, and any positive margin or high-risk features on final pathology should mandate further resection.
How do you determine which surgical procedure to perform?
Choosing which surgical procedure to perform on penile cancer patients can be challenging. The grade of the lesion, location, circumcision status, morphology, tumor quantity, and relationship to surrounding structures (urethra, corpora spongiosum, corpora cavernosa) must be taken in to consideration when determining the most appropriate surgical procedure. In addition, one also must consider patient age, comorbidities, body habitus, loss of penile length, and psychological ramifications.
An initial histologic diagnosis with a punch, excisional, or incisional biopsy is recommended to determine the risk of lymph node involvement (17). Further evaluation of the primary penile lesion can be performed with magnetic resonance imaging (MRI) or ultrasound to determine the depth of invasion. Lont et al. emphasized that physical examination alone is a reliable method to determine tumor size and predict corpus cavernosal infiltration with a high sensitivity (86%) and positive predictive value (100%); further imaging is warranted when physical examination is inconclusive (18). Another approach to penile cancer treatment is to start with penile-preserving surgery as primary treatment to allow more accurate staging—as biopsies can under-stage the disease and lead to insufficient treatment (19).
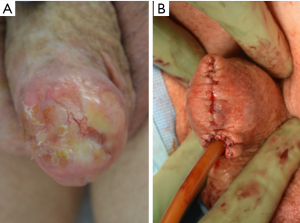
We prefer to biopsy the lesion first and then proceed to penile-preserving procedures to allow for complete excision of the primary tumor with maximal preservation of a functional and cosmetic penis. Penile-preserving procedures are indicated for Tis/Ta/T1 and some T2 tumors with favorable histology (11) (Figure 4). Established options include circumcision for preputial lesions, laser ablation, wide local excision, glans resurfacing, glansectomy, and Mohs micrographic surgery.
Circumcision
For lesions confined to the prepuce of an uncircumcised male, circumcision can have a multifactorial role in treating the disease, completely excising a previously biopsied primary lesion or serve as an initial staging procedure (19). Circumcision also allows for topical treatment of the glans, when indicated, and close clinical examination during follow-up visits. Circumcision can be used in conjunction with wide local excision and a portion of the disease-free shaft skin can be used to reconstruct glandular defects (Figure 5).
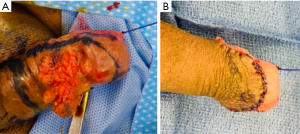
Circumcision in properly selected patients does not compromise overall survival but does carry an increased risk of local recurrence. These recurrences can be safely managed with repeat excision without the need to convert to partial or radical penectomy (20,21). However, this does necessitate closer follow-up; poor patient compliance is a contraindication.
Laser ablation
Laser ablation uses coherent electromagnetic radiation to destroy tissue. Available technologies include carbon dioxide (CO2) lasers (wavelength: 10,600 nm; tissue penetration: 0.1 mm) and neodymium-doped yttrium aluminum garnet (Nd:YAG) lasers (wavelength 1,064 nm; tissue penetration: 3–4 mm). A dilute preparation of acetic acid can be applied to areas of concern prior to ablation to induce aceto-whitening of tissues which tend to be associated with HPV infection—a sensitive but non-specific reaction (22).
The CO2 laser is efficiently absorbed by water, resulting in rapid vaporization of superficial tissue with some deeper necrosis due to photothermal coagulation of protein (23). For superficial lesions, the laser is swept over the tissue at a fixed distance and steady speed with 5 mm margins around the visible tumor.
In contrast, the Nd:YAG laser penetrates more deeply—causing more extensive photothermal coagulation and necrosis. This results in an adherent tissue coagulum which can be either excised or allowed to slough off on its own (24).
In both cases a smoke evacuator and appropriate personal protective equipment (PPE) must be used to minimize the risk of aerosolized HPV transmission to OR personnel (25). Circumcision is performed if not previously in order to facility treatment and monitoring. Local recurrence is common, up to 48%, but inguinal recurrence is rare with only 2% reported in pTa/Tis and 5% for pT1a primaries. While properly selected pT1b and pT2 can be treated with laser therapy, they require a staging inguinal procedure (26).
Wide local excision
Wide local excision is a class of procedures in which superficial lesions on the glans, prepuce, and penile shaft are excised with a small margin. Small defects can be closed primarily, while larger defects will require split or full thickness skin grafts (27). Full thickness grafts have historically produced better cosmesis with less tissue contraction. Some surgeons advocate for using a zig-zag incision to reduce circumferential contracture and 2-0 PDS tacking sutures to fix the graft to Buck’s fascia (28).
Glans resurfacing and glansectomy
The glans penis is the most common site of penile cancer with 50% of newly diagnosed lesions isolated to the glans and 80% isolated to the glans and prepuce (29). The least invasive treatment of these lesions is glans resurfacing. This involves surgical removal of the epithelial and subepithelial tissue, either in a wedge or from the entire glans. Deep frozen biopsies are taken from the underlying spongiosum—with special attention paid to areas below the visible tumor. A split-thickness skin graft, unmeshed and taken from non-hair bearing skin, is then applied and quilted in place with fine absorbable suture (30).
A recent 19 patient prospective cohort, the largest yet published, showed excellent cosmetic, functional, and oncologic outcomes with this technique, though no formal comparison with alternatives can be made (30).
Glansectomy is indicated for larger or more advanced lesions, including T2 lesions with urethral involvement isolated to the glans. A circumferential subcoronal incision is made and the glans is dissected off the tips of the corpora cavernosa, either above or below Buck’s fascia depending on the lesion location (27,31). Frozen sections from both the urethra and corporal bodies guide the extent of excision. Split-thickness skin grafts can be used to cover the corporal tips similar to the technique described above. Alternatively, a urethral advancement flap can be created by releasing the penile urethra to the penoscrotal junction in order to gain enough mobilization for 2 cm of urethral advancement. The urethra is widely spatulated along the ventral side, then secured to the tunica albuginea with absorbable suture (20,32,33).
Several other techniques for glans reconstruction have been described—including buccal mucosa graft augmentation, scrotal flaps, and myofascial flaps (34,35).
Mohs micrographic surgery
Mohs micrographic surgery is extensively used for treatment of cutaneous malignancies, especially in cosmetically sensitive areas. During Mohs, the bulk of the tumor is resected as in wide local excision but without a margin. Thin slices are taken from the resection bed and carefully examined microscopically by the operating surgeon and/or support staff. Additional smaller slices are taken from areas with residual disease until negative margins are achieved across the resection bed. Urethral involvement is typically managed with ventral meatotomy combined with urethrotomy to allow for circumferential tissue resection. Reconstruction can then be performed using methods described above to close the resulting defect.
The spongy nature of penile tissue, as well as difficulty detecting pre-malignant HPV infected cells on frozen section, has made application of this technique to penile cancer somewhat challenging. This has resulted in higher recurrence rates than reported for Mohs with other cutaneous malignancies, although comparable with other organ sparing penile techniques (36-38).
Management of inguinal nodes
Radical ILND has traditionally been associated with high morbidity, with early reports suggesting a near 100% complication rate. This has decreased with standardized reporting of surgical complications and improvements in technique and post-operative management. A 2009 review reported a major complication rate of 20–30% for radical resection (39). Complications may include hemorrhage, prolonged lymphatic secretion, lymphocele, cellulitis, wound dehiscence or necrosis. Concerns regarding morbidity may explain the apparent reluctance to perform an ILND , even if indicated by current guidelines. In 2011, Thuret at al. showed only ~30% adherence to National Cancer Institute guidelines which is concerning as delay has been shown to decrease survival (40). When comparing watchful waiting followed by lymphadenectomy at time of palpable disease with immediate lymphadenectomy in men with positive nodes, Kroon et al. found a 3-year CSS of 84% versus 35% in favor of immediate lymphadenectomy (7).
Upfront radical ILND is offered to patients with non-bulky palpable inguinal lymph nodes, whereas patients with bulky or initially unresectable nodal disease should referred to medical oncologist to consider neoadjuvant therapy prior to resection (41).
Patients with clinically negative nodes have been shown to harbor metastatic disease in anywhere from 11 to 62% of patients (42). While patients with early metastatic disease benefit from immediate resection, many patients with negative pathology might be exposed to a morbid procedure without benefit (7). Current guidelines stratify patients based on the primary lesion, with high risk tumors proceeding to invasive node assessment with either diagnostic sentinel lymph node biopsy (DLNB) or modified ILND (mILND). If positive on DLNB, the surgeon then proceeds to radical resection of the inguinal nodes (17). This allows patients with negative groins to be spared the morbidity of radical ILND without delaying care for those who will go on to develop palpable disease.
Radical ILND
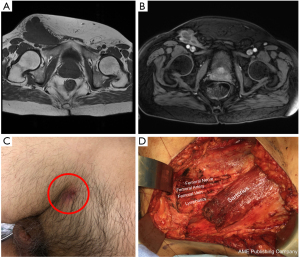
Radical inguinal lymphadenectomy remains standard of care for palpable, resectable node disease (43). This involves the removal of all lymphatic tissues in a quadrilateral area circumscribing the femoral triangle. This area is defined by 4 points: the anterior superior iliac spine, the superior margin of the inguinal canal, a point 20 cm inferior to the anterior superior iliac spine, and a point 15 cm inferior to the pubic tubercle (44). An incision is made 2 cm below and parallel to the inguinal ligament—extending the full width of the ultimate dissection. The key step in this surgery is dissecting generous skin flaps below Scarpa’s fascia from the superior to the inferior limits of the dissection. We recommend tagging the Scarpa’s fascia with silk sutures assist in the dissection. When dissection is carried superficial to Scarpa’s fascia, this may increase the risk of flap necrosis. The long saphenous vein is identified at the apex of the femoral triangle and ligated. Dissection is carried through the deep fascia of the thigh (fascia lata) from the Sartorius to the adductor longus muscle and into the femoral triangle. The femoral vessels are identified at the apex of the femoral triangle and used to guide the dissection proximally to the femoral canal, ligating perforating vessels as they are encountered. The saphenous vein is again ligated at its junction with the femoral vein. The femoral vessels are exposed and care is taken to avoid the lateral surface of the femoral artery to protect the femoral nerve. The sartorius muscle is commonly transposed to cover the femoral vessels to prevent hemorrhagic complications (44) (Figure 6). We also recommend resection of the overlying skin if it is fixed to the underlying tissue, cancer is eroding through the skin (Figure 6C), or previous biopsy was taken through this region. In addition, if there is a large skin defect present we employ negative pressure wound therapy versus grafts.
mILND
mILND was first described by Catalona in 1988. He noted that anatomical studies of lymphatic drainage showed a clear preference for the superiomedial nodes found along the superficial external pudendal and superficial epigastric vessels. Furthermore, there was little evidence for skip metastases without first involving these nodes, as well as those centrally within the fossa ovalis (43). As such, the modified template utilizes a shorter 10 cm incision made 2 cm below the inguinal ligament, starting at the pubic tubercle. An 8 cm superior flap and 6 cm inferior flap are created in similar fashion as described above. Deep nodes from the fossa ovalis, between the adductor longus and the lateral border of the femoral artery, are excised. The saphenous vein is preserved (5,43,44). If frozen specimens are positive, the procedure converted to a radical dissection.
Surgical complications following mILND are less common, with a modern series reporting no complications in 86% to 93% of dissections (45,46). Minor lymphedema remains the most commonly reported issue. During the postoperative period for both modified and radical lymphadenectomy we recommend lower extremity compression (>40 mmHg) stockings, referral to occupational therapy, and the use of drains until the output is less than 30 mL over a 48-hour period.
Dynamic sentinel lymph node biopsy
Dynamic sentinel lymph node biopsy is based on the same principle as the mILND—lymphatic spread of penile cancer is orderly and its absence in the proximal draining nodes excludes its presence in more distal nodes. DSLNB is typically performed at the time of penile surgery, though it can be delayed if there is ambiguity in the stage of the primary lesion (47). Radio-labelled 99mTc-nanocolloid is injected into the peritumoral tissue the day prior to the procedure and single-photon emission computed tomography with computed tomography (SPECT/CT) images are captured to aid in surgical planning. Shortly before the procedure, blue dye is injected in the same manner. Intraoperatively, a handheld gamma probe, in conjunction with visualization of the blue dye, are used to identify sentinel nodes (48). Studies on DSLNB have consistently emphasized the need for experienced practitioners in high volume centers to minimize false negative biopsies (49).
Recent advances have resulted in improved detection outcomes. Routine fine needle aspiration of suspicious nodes seen on ultrasound allows for the detection of extensively infiltrated nodes with obstructed lymphatic drainage. The addition of routine ultrasound with or without FNA prior to DSLNB resulted in a 6% false negative rate and a similar complication rate (49,50). It has recently been shown that a new hybrid radioactive and fluorescent indocyanine green-99mTc-nanocolloid resulted in marked improvement in sentinel node visualization compared to the traditional blue dye (51).
Video endoscopic inguinal lymphadenectomy (VEIL)
Minimally invasive surgical techniques have been widely adopted in the last two decades in effort to reduce morbidity. VEIL was first described by Bishoff et al. in 2003 (52,53). Since that time, several series have been published detailing their results with the technique and robotic-assisted variants. The initial incision for the camera port is made just distal to the apex of the femoral triangle below Camper’s fascia. A combination of sharp and blunt dissection is used to develop the space needed for port placement. Two working ports are placed laterally and superiorly to the camera outside the medial and lateral edges of the planned dissection to create a triangular array with sufficient space to prevent instrument clash. If done robotically, an assistant port can be placed between the camera and the medial working port. Insufflation is established with CO2 at 10–12 mmHg. Flap thickness is controlled by palpating between the instrument and hand. Superficial nodes are excised from proximal to distal off the iliac spine and the pubic tubercle, sparing the saphenous vein. The deep nodes are then excised working distal to proximal, from the apex of the femoral triangle to the sapheno-femoral junction. Node packets are removed with a laparoscopic bag (52,54).
Early data with this approach has been promising. A 2017 non-randomized prospective study published compared 51 robotic assisted-VEIL (RA-VEIL) to 100 open lymph node dissection (OLND) and found significantly lower rates of major complications (2% vs. 17%). Rates of minor complications—including lymphocele, surgical site infection (SSI), cellulitis, and non-debilitating leg edema—were similar, experienced by more than 75% of patients in both groups. While not controlled, patients were comparable in terms of comorbidities and disease status. Furthermore, they found equivalent nodal yields (12.5 vs. 13) and pathologic stage with no recurrence in either group at 40-month follow-up. However, RA-VEIL had increased operative time (55).
Another non-randomized prospective study from 2017 compared OLND with VEIL in 42 patients with similar findings: lower Clavien-Dindo Grade III and above wound complications (6% vs. 68%) with equivalent lymph node yields and no groin recurrences in either groups (54).
Conclusions
Treatment for penile cancer continues to evolve as new technologies become available. Surgery remains the cornerstone for treating the primary lesion and inguinal lymph nodes with emphasis placed on the preservation of function without compromising oncologic control. Advances in imaging and diagnostics have been critical to this endeavor both in regards characterizing the primary lesion and better identifying metastatic inguinal disease (56). Molecular and genomic profiling studies have furthered our understanding of tumor biology, with multiple targeted-therapy trials in progress (57). Still, the rarity of the disease limits our ability to conduct randomized controlled trials and there remain significant knowledge gaps of how best to treat this potentially debilitating disease. The International Penile Advanced Cancer Trial (InPACT) is currently accruing patients and aims to answer several key questions regarding management of nodal disease. Patients with clinical evidence of inguinal lymph node metastases are randomized to ILND with either neoadjuvant chemotherapy, chemo-radiotherapy, or no neoadjuvant therapy, with a further high risk subset randomized to receive possible prophylactic pelvic lymph node dissection (58). Further studies and international collaborations like InPACT are needed to improve our understanding and treatment of this disease.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Simon P. Kim) for the series “Surgical Management of Genitourinary Malignancies” published in AME Medical Journal. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-20-159/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-159/coif). The series “Surgical Management of Genitourinary Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Christodoulidou M, Sahdev V, Houssein S, et al. Epidemiology of penile cancer. Curr Probl Cancer 2015;39:126-36. [Crossref] [PubMed]
- Douglawi A, Masterson TA. Penile cancer epidemiology and risk factors: a contemporary review. Curr Opin Urol 2019;29:145-9. [Crossref] [PubMed]
- Pekala KR, Pelzman D, Theisen KM, et al. The Prevalence of Penile Cancer in Patients With Adult Acquired Buried Penis. Urology 2019;133:229-33. [Crossref] [PubMed]
- Harder T, Wichmann O, Klug SJ, et al. Efficacy, effectiveness and safety of vaccination against human papillomavirus in males: a systematic review. BMC Med 2018;16:110. [Crossref] [PubMed]
- Leone A, Diorio GJ, Pettaway C, et al. Contemporary management of patients with penile cancer and lymph node metastasis. Nat Rev Urol 2017;14:335-47. [Crossref] [PubMed]
- Srinivas V, Morse MJ, Herr HW, et al. Penile cancer: relation of extent of nodal metastasis to survival. J Urol 1987;137:880-2. [Crossref] [PubMed]
- Kroon BK, Horenblas S, Lont AP, et al. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J Urol 2005;173:816-9. [Crossref] [PubMed]
- Gursel EO, Georgountzos C, Uson AC, et al. Penile cancer. Urology 1973;1:569-78. [Crossref] [PubMed]
- Matulewicz RS, Flum AS, Helenowski I, et al. Centralization of Penile Cancer Management in the United States: A Combined Analysis of the American Board of Urology and National Cancer Data Base. Urology 2016;90:82-8. [Crossref] [PubMed]
- Joseph A. Smith J, Howards SS, McGuire EJ, et al. Hinman's Atlas of Urologic Surgery, Third Edition Philadelphia: Elsevier Saunders; 2012.
- Sharpe D, Angermeier K. Surgery of Penile and Urethral Carcinoma. Campbells Walsh Urology; 2010.
- Agrawal A, Pai D, Ananthakrishnan N, et al. The histological extent of the local spread of carcinoma of the penis and its therapeutic implications. BJU Int 2000;85:299-301. [Crossref] [PubMed]
- Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU Int 2005;96:1040-3. [Crossref] [PubMed]
- Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. J Urol 2012;188:803-8. [Crossref] [PubMed]
- Sri D, Sujenthiran A, Lam W, et al. A study into the association between local recurrence rates and surgical resection margins in organ-sparing surgery for penile squamous cell cancer. BJU Int 2018;122:576-82. [Crossref] [PubMed]
- Hakenberg OW, Comperat EM, Minhas S, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol 2015;67:142-50. [Crossref] [PubMed]
- Network NCC. Penile Cancer (Version 1.2020). Available online: https://www.nccn.org/professionals/physician_gls/pdf/penile.pdf. Accessed 3/1/2020.
- Lont AP, Besnard AP, Gallee MP, et al. A comparison of physical examination and imaging in determining the extent of primary penile carcinoma. BJU Int 2003;91:493-5. [Crossref] [PubMed]
- Mahesan T, Hegarty PK, Watkin NA. Advances in Penile-Preserving Surgical Approaches in the Management of Penile Tumors. Urol Clin North Am 2016;43:427-34. [Crossref] [PubMed]
- Kamel MH, Bissada N, Warford R, et al. Organ Sparing Surgery for Penile Cancer: A Systematic Review. J Urol 2017;198:770-9. [Crossref] [PubMed]
- Lont AP, Gallee MP, Meinhardt W, et al. Penis conserving treatment for T1 and T2 penile carcinoma: clinical implications of a local recurrence. J Urol 2006;176:575-80; discussion 580. [Crossref] [PubMed]
- Marina OC, Sanders CK, Mourant JR. Effects of acetic acid on light scattering from cells. J Biomed Opt 2012;17:085002-1. [Crossref] [PubMed]
- Baleg SM, Bidin N, Suan LP, et al. The effect of CO2 laser treatment on skin tissue. J Cosmet Dermatol 2015;14:246-53. [Crossref] [PubMed]
- Schlenker B, Tilki D, Seitz M, et al. Organ-preserving neodymium-yttrium-aluminium-garnet laser therapy for penile carcinoma: a long-term follow-up. BJU Int 2010;106:786-90. [Crossref] [PubMed]
- Manson LT, Damrose EJ. Does exposure to laser plume place the surgeon at high risk for acquiring clinical human papillomavirus infection? Laryngoscope 2013;123:1319-20. [Crossref] [PubMed]
- Tang DH, Yan S, Ottenhof SR, et al. Laser ablation as monotherapy for penile squamous cell carcinoma: A multi-center cohort analysis. Urol Oncol 2018;36:147-52. [Crossref] [PubMed]
- Burnett AL. Penile preserving and reconstructive surgery in the management of penile cancer. Nat Rev Urol 2016;13:249-57. [Crossref] [PubMed]
- Monn MF, Socas J, Mellon MJ. The Use of Full Thickness Skin Graft Phalloplasty During Adult Acquired Buried Penis Repair. Urology 2019;129:223-7. [Crossref] [PubMed]
- Arya M, Kalsi J, Kelly J, et al. Malignant and premalignant lesions of the penis. BMJ 2013;346:f1149. [Crossref] [PubMed]
- O'Kelly F, Lonergan P, Lundon D, et al. A Prospective Study of Total Glans Resurfacing for Localized Penile Cancer to Maximize Oncologic and Functional Outcomes in a Tertiary Referral Network. J Urol 2017;197:1258-63. [Crossref] [PubMed]
- Parnham AS, Albersen M, Sahdev V, et al. Glansectomy and Split-thickness Skin Graft for Penile Cancer. Eur Urol 2018;73:284-9. [Crossref] [PubMed]
- Belinky JJ, Cheliz GM, Graziano CA, et al. Glanuloplasty with urethral flap after partial penectomy. J Urol 2011;185:204-6. [Crossref] [PubMed]
- Gulino G, Sasso F, Falabella R, et al. Distal urethral reconstruction of the glans for penile carcinoma: results of a novel technique at 1-year of followup. J Urol 2007;178:941-4. [Crossref] [PubMed]
- Mazza ON, Cheliz GM. Glanuloplasty with scrotal flap for partial penectomy. J Urol 2001;166:887-9. [Crossref] [PubMed]
- Giovanny A, Wahyudi I, Rodjani A. Neo-glans reconstruction after glans amputation during circumcision using autologous buccal mucosal graft. Urol Case Rep 2018;18:11-3. [Crossref] [PubMed]
- Shindel AW, Mann MW, Lev RY, et al. Mohs micrographic surgery for penile cancer: management and long-term followup. J Urol 2007;178:1980-5. [Crossref] [PubMed]
- Mohs FE, Snow SN, Larson PO. Mohs micrographic surgery for penile tumors. Urol Clin North Am 1992;19:291-304. [Crossref] [PubMed]
- Machan M, Brodland D, Zitelli J. Penile Squamous Cell Carcinoma: Penis-Preserving Treatment With Mohs Micrographic Surgery. Dermatol Surg 2016;42:936-44. [Crossref] [PubMed]
- Spiess PE, Hernandez MS, Pettaway CA. Contemporary inguinal lymph node dissection: minimizing complications. World J Urol 2009;27:205-12. [Crossref] [PubMed]
- Thuret R, Sun M, Lughezzani G, et al. A contemporary population-based assessment of the rate of lymph node dissection for penile carcinoma. Ann Surg Oncol 2011;18:439-46. [Crossref] [PubMed]
- Pettaway CA, Pagliaro L, Theodore C, et al. Treatment of visceral, unresectable, or bulky/unresectable regional metastases of penile cancer. Urology 2010;76:S58-65. [Crossref] [PubMed]
- Slaton JW, Morgenstern N, Levy DA, et al. Tumor stage, vascular invasion and the percentage of poorly differentiated cancer: independent prognosticators for inguinal lymph node metastasis in penile squamous cancer. J Urol 2001;165:1138-42. [Crossref] [PubMed]
- Catalona WJ. Modified inguinal lymphadenectomy for carcinoma of the penis with preservation of saphenous veins: technique and preliminary results. J Urol 1988;140:306-10. [Crossref] [PubMed]
- Ercole CE, Pow-Sang JM, Spiess PE. Update in the surgical principles and therapeutic outcomes of inguinal lymph node dissection for penile cancer. Urol Oncol 2013;31:505-16. [Crossref] [PubMed]
- Bouchot O, Rigaud J, Maillet F, et al. Morbidity of inguinal lymphadenectomy for invasive penile carcinoma. Eur Urol 2004;45:761-5; discussion 765-6. [Crossref] [PubMed]
- Yao K, Tu H, Li YH, et al. Modified technique of radical inguinal lymphadenectomy for penile carcinoma: morbidity and outcome. J Urol 2010;184:546-52. [Crossref] [PubMed]
- Omorphos S, Saad Z, Arya M, et al. Feasibility of performing dynamic sentinel lymph node biopsy as a delayed procedure in penile cancer. World J Urol 2016;34:329-35. [Crossref] [PubMed]
- Leijte JA, Kroon BK, Valdes Olmos RA, et al. Reliability and safety of current dynamic sentinel node biopsy for penile carcinoma. Eur Urol 2007;52:170-7. [Crossref] [PubMed]
- Kamel MH, Khalil MI, Davis R, et al. Management of the Clinically Negative (cN0) Groin Penile Cancer Patient A Review. Urology 2019;131:5-13. [Crossref] [PubMed]
- Lam W, Alnajjar HM, La-Touche S, et al. Dynamic sentinel lymph node biopsy in patients with invasive squamous cell carcinoma of the penis: a prospective study of the long-term outcome of 500 inguinal basins assessed at a single institution. Eur Urol 2013;63:657-63. [Crossref] [PubMed]
- Brouwer OR, van den Berg NS, Matheron HM, et al. A hybrid radioactive and fluorescent tracer for sentinel node biopsy in penile carcinoma as a potential replacement for blue dye. Eur Urol 2014;65:600-9. [Crossref] [PubMed]
- Josephson DY, Jacobsohn KM, Link BA, et al. Robotic-assisted endoscopic inguinal lymphadenectomy. Urology 2009;73:167-70; discussion 170-1. [Crossref] [PubMed]
- Bishoff JA, Lackland AFB, Basler JW, et al. Endoscopy subcutaneous modified inguinal limph node dissection (ESMIL) for squamous cell carcinoma of the penis. J Urol 2003;169:78.
- Kumar V, Sethia KK. Prospective study comparing video-endoscopic radical inguinal lymph node dissection (VEILND) with open radical ILND (OILND) for penile cancer over an 8-year period. BJU Int 2017;119:530-4. [Crossref] [PubMed]
- Singh A, Jaipuria J, Goel A, et al. Comparing Outcomes of Robotic and Open Inguinal Lymph Node Dissection in Patients with Carcinoma of the Penis. J Urol 2018;199:1518-25. [Crossref] [PubMed]
- de Vries HM, Brouwer OR, Heijmink S, et al. Recent developments in penile cancer imaging. Curr Opin Urol 2019;29:150-5. [Crossref] [PubMed]
- Peyraud F, Allenet C, Gross-Goupil M, et al. Current management and future perspectives of penile cancer: An updated review. Cancer Treat Rev 2020;90:102087. [Crossref] [PubMed]
- Canter DJ, Nicholson S, Watkin N, et al. The International Penile Advanced Cancer Trial (InPACT): Rationale and Current Status. Eur Urol Focus 2019;5:706-9. [Crossref] [PubMed]
Cite this article as: Coddington ND, Redger KD, Higuchi TT. Surgical principles of penile cancer for penectomy and inguinal lymph node dissection: a narrative review. AME Med J 2021;6:29.


