
Clinicopathological and genetic features of anastomosing haemangioma of the kidney
Introduction
Anastomosing haemangioma is a benign neoplasm composed of anastomosing sinusoidal capillary-sized vessels (1). Initially described in the genitourinary system, the tumour has since been reported in various anatomical sites including the ovary (2,3), adrenal gland, liver, mesentery, testis, spermatic cord, para-aortic tissue, perirenal tissue (3), small and large bowel (4), paraspinal soft tissues, anterior mediastinum, uterine cornu, upper arm and infundibular pelvic ligament (5). Because anastomosing haemangioma mimics primary renal angiosarcoma—a known diagnostic pitfall with implications on management and outcome—there is a need to better understand this neoplasm.
For this narrative review, we extensively searched the English literature for all cases of anastomosing haemangioma of the kidney. This article summarizes state-of-the-art knowledge on the epidemiology, clinical and pathologic features of anastomosing haemangioma of the kidney as well as treatment, prognosis, and differential diagnosis.
Epidemiology
Anastomosing haemangioma of the kidney is extremely rare. To date, approximately 75 cases have been reported in the literature, mostly as case reports or series (1-25). The mean age of patients at diagnosis is 49 years (range, 10–83 years) (6,11,12). There is a slight male predilection with a male-to-female ratio of 2:1 (6). There is no known racial association.
Aetiology
The aetiology is not known. Unlike haemangioma of the bladder, there is no established association with cutaneous haemangiomas, tuberous sclerosis, Klippel-Trenaunay syndrome and Sturge-Weber syndrome (13-15). However, anastomosing haemangioma has been reported in patients with end stage renal disease (ESRD). In a study of 20 nephrectomies from 16 patients with ESRD, there were 36 renal haemangiomas, and all except one were anastomosing haemangiomas (16). Despite the association between ESRD and anastomosing haemangioma, a clear pathogenetic link is yet to be established and the occurrence of anastomosing haemangioma is not unique to ESRD.
Clinical features
Most anastomosing haemangiomas of the kidney are discovered incidentally during imaging or examination of nephrectomies for other renal neoplasms (6). There are no symptoms unique to this tumour. Patients may present with haematuria, abdominal pain, abdominal mass (6) and lower urinary tract symptoms (LUTs) (13). Rarely, patients may present with retroperitoneal haematoma (16,17) or life-threatening haemorrhage (17,18).
This tumour may co-exist with other renal neoplasms including papillary adenoma (16,19,20), metanephric adenoma, papillary renal cell carcinoma, clear cell renal cell carcinoma (21) and acquired cystic disease-associated renal cell carcinoma (16).
There are no imaging features unique to anastomosing haemangiomas. CT imaging shows well-circumscribed, hyperdense and heterogeneous masses with avid post-contrast enhancement (3,22). The considerable overlap of imaging features with other renal tumours makes image-based diagnosis challenging.
Pathology
Grossly, the tumours are well circumscribed masses. They are mostly unencapsulated with fleshy and spongy mahogany brown or haemorrhagic cut surface. The tumours are often small, averaging 2.2 cm in diameter (range, 0.1–12 cm) (6,12). Typically, anastomosing haemangiomas are unilateral and solitary lesions. However, bilateral (2,16,19) and multifocal tumours have been described (16,21). Rarely, gross extension of tumours into the renal vein (1,2,23) or segmental branches of the renal vein (16,24) may be seen. Additionally, the tumours may invade renal sinus fat (16) and perinephric fat (1).
Microscopically, the tumours are well demarcated with a vaguely lobular architecture on low power examination. They are composed of anastomosing sinusoidal capillary-sized vessels lined by a single layer of bland endothelial cells (shown in Figure 1). The vascular channels are separated and supported by loose stroma.
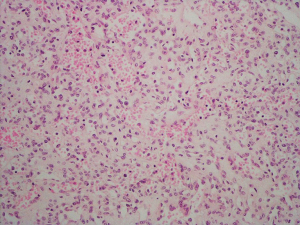
Extramedullary haematopoiesis is a common feature in these tumours (1,2,16,25). Rarely, some tumours may contain hyaline globules reminiscent of those seen in Kaposi’s sarcoma (1,2,16). Tumours may feature areas with sclerosis, fibrin thrombi, haemorrhage (1,13,20) and minimal inflammatory infiltrate composed of lymphocytes (1,2). Typically, anastomosing haemangiomas lack features of malignancy including abnormal mitotic figures, marked cytologic atypia, endothelial multilayering and papillary tufting (6).
The lesional cells are positive for CD31 (shown in Figure 2), CD34 (shown in Figure 3), ERG, FLI1 and factor VIII-related antigen. The supporting stromal cells express smooth muscle actin. The lesional cells are negative for HHV-8, a marker for Kaposi’s sarcoma (6).
Genetics
Anastomosing haemangiomas harbour recurrent somatic mutations in the GNAQ gene and its paralogue, GNA14 (26,27). GNAQ encodes Gαq protein (guanine nucleotide-binding protein G(q) subunit alpha). The protein is part of the heterotrimeric G protein complex. Heterotrimeric G proteins are signal transducers that relay diverse extracellular signals received by G protein coupled receptors (GPCRs) to downstream intracellular effectors (28,29). The heterotrimeric G protein complex comprises 3 subunits: α, β and γ. Gαq is the alpha subunit of the complex and in its inactive state, is bound to guanine diphosphate (GDP) (29).
Upon activation of GPCRs by ligand binding, Gαq releases GDP in exchange for guanine triphosphate (GTP), separates from the complex and activates downstream signalling pathways that are important in the regulation of cell proliferation, survival, protein synthesis and development of blood vessels (30). This signalling is turned off by intrinsic GTPase activity which converts GTP to GDP. Activating somatic mutations in GNAQ impairs the ability of the altered Gαq protein to return to its inactive state. As a result, Gαq remains in its active GTP-bound state and downstream signalling pathways are constantly turned on (26,27,29-31).
Of the 15 anastomosing haemangiomas studied using next generation DNA sequencing, 9 harboured GNAQ p.Q209H mutation; 1 had GNAQ p.Q209L mutation; 4 showed GNA14 p.Q205L mutation; and 1 had neither GNAQ nor GNA14 mutation (26,27). No other pathogenic mutations particularly mutations seen in angiosarcomas were identified in these studies (26). Q205 in GNA14 is a hotspot and the equivalent residue to Q209 in GNAQ (27).
Interestingly, these mutations are not unique to anastomosing haemangiomas. Mutations in GNAQ and its paralogues including GNA11 and GNA14 have been identified in hepatic small vessel neoplasm (HSVN) (32), congenital haemangiomas (33), non-syndromic port wine stains, Sturge-Weber syndrome (34), cherry haemangiomas (35) and melanocytic lesions such as primary uveal melanoma and blue nevi (29).
Prognosis
The vast majority of anastomosing haemangioma of the kidney were treated with radical nephrectomy and only few cases were treated with partial nephrectomy (Table 1). This probably reflects a tendency to overtreat renal anastomosing haemangiomas, probably because most cases have a preoperative diagnosis of renal cell cancer.
Table 1
| Author | PMID/Ref No | Treatment | Follow-up (months) | Outcome |
|---|---|---|---|---|
| Wetherell et al. | 23250038 | Nephrectomy | 1 | DFUD |
| Omiyale et al. | 26435872 | Nephrectomy | 10 | NED |
| Montgomery & Epstein | 19606014 | Nephrectomy | 12 | NED |
| Montgomery & Epstein | 19606014 | Nephrectomy | 36 | NED |
| Montgomery & Epstein | 19606014 | Nephrectomy | NA | NA |
| Montgomery & Epstein | 19606014 | Excision | 8 | NED |
| Brown et al. | 20534992 | Nephrectomy | 72 | NED |
| Brown et al. | 20534992 | Nephrectomy | 24 | NED |
| Brown et al. | 20534992 | Partial nephrectomy | NA | NA |
| Brown et al. | 20534992 | Nephrectomy | 24 | NED |
| Brown et al. | 20534992 | Nephrectomy | NA | NA |
| Tran & Pernicone | 24578924 | Nephrectomy | NA | NA |
| Mehta et al. | 23090628 | Nephrectomy | 3 | NED |
| Mehta et al. | 23090628 | Nephrectomy | 12 | NED |
| Mehta et al. | 23090628 | Nephrectomy | 3 | NED |
| Tahir & Folwell | 25041271 | Nephrectomy | 1 | NED |
| Kryvenko et al. | 24548339 | Nephrectomy | NA | NA |
| Kryvenko et al. | 24548339 | Nephrectomy | NA | NA |
| Kryvenko et al. | 24548339 | Nephrectomy | NA | NA |
| Kryvenko et al. | 24548339 | Nephrectomy | NA | NA |
| Kryvenko et al. | 24548339 | Nephrectomy | NA | NA |
| Kryvenko et al. | 24548339 | Nephrectomy | NA | NA |
| Kryvenko et al. | 24548339 | Nephrectomy | NA | NA |
| Kryvenko et al. | 24548339 | Nephrectomy | NA | NA |
| Kryvenko et al. | 24548339 | Nephrectomy | NA | NA |
| Kryvenko et al. | 24548339 | Nephrectomy | NA | NA |
| Kryvenko et al. | 24548339 | Nephrectomy | NA | NA |
| Kryvenko et al. | 24548339 | Nephrectomy | NA | NA |
| Pantelides et al. | (7) | Nephrectomy | 6 | NED |
| Heidegger et al. | 24650180 | Nephrectomy | 156 | NED |
| Buttner et al. | 23020314 | Nephrectomy | NA | NA |
| Buttner et al. | 23020314 | Nephrectomy | NA | NA |
| Buttner et al. | 23020314 | Nephrectomy | NA | NA |
| Buttner et al. | 23020314 | Nephrectomy | NA | NA |
| Buttner et al. | 23020314 | Nephrectomy | NA | NA |
| Buttner et al. | 23020314 | Nephrectomy | NA | NA |
| Buttner et al. | 23020314 | Nephrectomy | NA | NA |
| Buttner et al. | 23020314 | Nephrectomy | NA | NA |
| Al-Maghrabi & Al-Rashed | 28286335 | Partial nephrectomy | 12 | NED |
| Caballes et al. | 30369288 | Nephrectomy | 18 | NED |
| Kryvenko et al. | 21846922 | Nephrectomy | 7 | NED |
| Kryvenko et al. | 21846922 | Nephrectomy | 6 | NED |
| Kryvenko et al. | 21846922 | Nephrectomy | 3 | NED |
| Kryvenko et al. | 21846922 | Nephrectomy | 122 | NED |
| Cheon et al. | 29962849 | Nephrectomy | 6 | NED |
| Abboudi et al. | 29279869 | Nephrectomy | <1 | NED |
| Silva et al. | 28727378 | Resection | NA | NA |
| Berker et al. | 28436289 | Partial nephrectomy | 10 | NED |
| Berker et al. | 28436289 | Nephrectomy | 4 | NED |
| Bean et al. | 28084343 | Nephrectomy | 9 | NED |
| Bean et al. | 28084343 | Nephrectomy | 84 | NED |
| Bean et al. | 28084343 | Nephrectomy | 107 | NED |
| Zhang et al. | 25973131 | Partial nephrectomy | 16 | NED |
| Perdiki et al. | 28118845 | Partial nephrectomy | 25 | NED |
| Perdiki et al. | 28118845 | Nephrectomy | 14 | NED |
| Chou et al. | 23816823 | Nephrectomy | 8 | NED |
| Chou et al. | 23816823 | Nephrectomy | 14 | NED |
| Zhao et al. | 23573324 | Nephrectomy | 12 | NED |
| Tao et al. | 25102914 | Nephrectomy | 21 | NED |
| Downes et al. | (8) | Nephrectomy | NA | NA |
| Downes et al. | (8) | Biopsy | NA | NA |
| Chandran et al. | (9) | Nephrectomy | NA | NA |
| Cha et al. | (10) | Nephrectomy | 5 | NED |
| Lee et al. | 11752931 | Nephrectomy | NA | NA |
| Memmedoglu & Musayev | 26623154 | Nephrectomy | 12 | NED |
| Memmedoglu & Musayev | 26623154 | Nephrectomy | 12 | NED |
| O’Neil et al. | 26960722 | NA | NA | NA |
| O’Neil et al. | 26960722 | NA | NA | NA |
| O’Neil et al. | 26960722 | NA | NA | NA |
| O’Neil et al. | 26960722 | NA | NA | NA |
| O’Neil et al. | 26960722 | NA | NA | NA |
| O’Neil et al. | 26960722 | NA | NA | NA |
| O’Neil et al. | 26960722 | NA | NA | NA |
| Johnstone et al. | 32111399 | Nephrectomy | NA | NA |
| Manohar et al. | 32317537 | Nephrectomy | 24 | NED |
NA, not available; NED, no evidence of disease; DFUD, died from unrelated disease.
Of the 75 cases described in the literature, follow-up data were available for 38 patients (50.7%). The mean follow-up time was 24.8 months (range, <1–156 months). One patient died from unrelated disease—embolic cerebrovascular event (13). There was no evidence of recurrence, metastasis or tumour-related death (Table 1).
Differential diagnosis
Anastomosing haemangiomas must be distinguished from primary angiosarcoma of the kidney, Kaposi’s sarcoma, intravascular papillary endothelial hyperplasia (IPEH) and angiomyolipoma (Table 2).
Table 2
| Differential diagnosis | Histological findings | Immunohistochemistry | Prognosis |
|---|---|---|---|
| Anastomosing haemangioma | Anastomosing capillary-sized blood vessels, single layer of hobnail endothelial cells, no atypia, extramedullary haematopoiesis | ERG+, CD31+, CD34+, FLI1+, HHV8− | Excellent prognosis, no metastasis and recurrence |
| Angiosarcoma | Anastomosing blood vessels, endothelial cell multilayering and papillary tufting, atypia, abnormal mitotic figures | ERG+, CD31+, CD34+, FLI1+, HHV8− | Highly aggressive tumour with limited response to surgery, chemotherapy and radiotherapy |
| Angiomyolipoma (AML) | Variable mixture of mature adipose tissue, poorly organised thick-walled blood vessels and smooth muscle | HMB45+, Melan A+, SMA+, Calponin+ | Classic AMLs are benign with an excellent prognosis |
| Epithelioid AMLs can show aggressive behaviour | |||
| Kaposi sarcoma | Thin-walled blood vessels, slit-like spaces containing extravasated red blood cells, spindled cells, DPAS-positive hyaline globules, plasma cells | HHV8+, ERG+, CD31+, CD34+, FLI1+ | Outcome depends on the extent of disease and visceral involvement |
| Intravascular papillary endothelial hyperplasia (Masson’s tumour) | Usually arises within a blood vessel, papillary formations, organizing thrombi, exuberant endothelial proliferation, no atypia | ERG+, CD31+, CD34+, FLI1+, HHV8− | Considered a benign and reactive lesion with an excellent prognosis |
Primary renal angiosarcoma is a key differential diagnosis. It shares similar clinical features with anastomosing haemangioma including abdominal mass, haematuria, flank pain and rarely, retroperitoneal haematoma. The imaging features of both tumours are non-specific. They also share similar anastomosing pattern on histology and are positive for CD34, CD31, ERG, FLI1 and factor VIII-related antigen (36).
Unlike anastomosing haemangiomas, primary renal angiosarcomas are malignant vascular tumours. Most renal angiosarcomas occur in patients in their 6th or 7th decade of life, although a wide age range (24–95 years) has been reported (36). They are typically symptomatic and frequently metastasise to various anatomical sites including the liver, lungs, bone, skin, spleen, and lymph nodes. Angiosarcomas are usually large and comprises capillary-sized blood vessels lined by malignant endothelial cells. The neoplastic cells are pleomorphic with hyperchromatic nuclei. Multilayering, abnormal mitotic figures and necrosis are common features. The prognosis of primary angiosarcoma of the kidney is poor despite treatment with surgery, chemotherapy and radiotherapy (36).
Kaposi’s sarcoma is a vascular neoplasm associated with human herpesvirus-8 (HHV-8). Clinically, four variants have been described including classic, endemic, AIDS-associated, and iatrogenic. This tumour is extremely rare in the kidney (37). Microscopically, Kaposi’s sarcoma consists of thin-walled blood vessels, spindled cells, lymphoplasmacytic infiltrate, slit-like spaces containing extravasated red blood cells and DPAS-positive hyaline globules. Hemosiderin deposits may be present. Common to both Kaposi’s sarcoma and anastomosing haemangioma are the presence of thin-walled blood vessels, hyaline globules (although more frequently seen in Kaposi’s sarcoma), and the expression of vascular markers (CD31 and CD34). However, Kaposi sarcoma can be confirmed by the expression of HHV-8-associated protein LANA-1 (6). The outcome is related to the extent of disease and visceral involvement (37).
Intravascular papillary endothelial hyperplasia (Masson’s tumour) is a reactive lesion that commonly occurs within the extremities and head and neck. Rare cases have been reported in the kidney (38). The lesion is characterized by organizing thrombi, prominent papillary structures with hyalinised or fibrous stalks, and hyperplastic endothelial cells, often within the lumen of a large blood vessel, but also in haematomas, or haemangiomas. There are no mitotic figures, atypia or necrosis. These lesions are benign with an excellent prognosis (6,38).
Because anastomosing haemangioma may show fatty changes, they must be distinguished from angiomyolipoma (AML) (39). Angiomyolipoma is composed of thick-walled poorly organized blood vessels, mature adipose tissue and smooth muscle. Unlike anastomosing haemangioma, angiomyolipoma is characterized by the co-expression of smooth muscle markers (smooth muscle actin and calponin) and melanocytic markers (Melan-A, HMB-45 and microphthalmia transcription factor) (6). Classic AMLs are benign; however, epithelioid AMLs may show aggressive behaviour including recurrence and metastasis (40).
Conclusion
Anastomosing haemangiomas of the kidney share similar clinical and imaging features with other renal neoplasms thus making them radiologically indistinguishable. They mimic primary renal angiosarcomas which must be considered as a differential diagnosis and ruled out. Unlike primary renal angiosarcoma with a poor prognosis, anastomosing haemangioma is benign and has an excellent prognosis with no recurrent or metastatic disease during follow-up. Definitive diagnosis requires pathologic assessment. Awareness of this rare tumour and the recognition of its benign clinical course should allow for accurate diagnosis and consideration for nephron-sparing surgery.
Learning points
- Anastomosing haemangioma of the kidney is a rare tumour.
- The tumour is characterized by anastomosing capillary-sized blood vessels lined by bland endothelial cells.
- Most anastomosing haemangiomas are discovered incidentally.
- Anastomosing haemangioma can mimic other vascular tumours of the kidney, particularly primary renal angiosarcomas.
- Anastomosing haemangioma harbours recurrent somatic mutations in the GNAQ gene and its paralogue, GNA14.
- Histopathology is the gold standard for diagnosis.
- Anastomosing haemangiomas have an excellent prognosis.
Acknowledgments
We would like to thank Philip Brown and Alexandra Roche for proofreading this article.
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-181/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Montgomery E, Epstein JI. Anastomosing hemangioma of the genitourinary tract: a lesion mimicking angiosarcoma. Am J Surg Pathol 2009;33:1364-9. [Crossref] [PubMed]
- Kryvenko ON, Gupta NS, Meier FA, et al. Anastomosing hemangioma of the genitourinary system: eight cases in the kidney and ovary with immunohistochemical and ultrastructural analysis. Am J Clin Pathol 2011;136:450-7. [Crossref] [PubMed]
- O’Neill AC, Craig JW, Silverman SG, et al. Anastomosing hemangiomas: locations of occurrence, imaging features and diagnosis with percutaneous biopsy. Abdom Radiol (NY) 2016;41:1325-32. [Crossref] [PubMed]
- Lin J, Biggie J, Ulbright TM, et al. Anastomosing hemangioma of the liver and gastrointestinal tract: an unusual variant histologically mimicking angiosarcoma. Am J Surg Pathol 2013;37:1761-5. [Crossref] [PubMed]
- John I, Folpe AL. Anastomosing hemangiomas arising in unusual locations: a clinicopathologic study of 17 soft tissue cases showing a predilection for the paraspinal region. Am J Surg Pathol 2016;40:1084-9. [Crossref] [PubMed]
- Omiyale AO. Anastomosing haemangioma of the kidney: a literature review of a rare morphological variant of haemangioma. Ann Transl Med 2015;3:151. [PubMed]
- Pantelides NM, Agrawal S, Mawson I, et al. An Anastomosing Haemangioma: A Rare Vascular Tumour Presenting as a Solid Renal Mass. Br J Med Surg Urol 2012;5:84-86. [Crossref]
- Downes MR, Dickson BC, Cheung CC. Anastomosing haemangioma of kidney: morphologic features and diagnostic considerations of an unusual vasoformative tumour Diagn Histopathol 2014;20:208-12. [Crossref]
- Chandran N, Kannan MS, Veeramani M. Renal anastomosing hemangioma: a diagnosis to ponder Indian J Transplant 2019;13:59-61.
- Cha JS, Jeong YB, Kim HJ. Anastomosing hemangioma mimicking renal cell carcinoma. Korean J Urol Oncol 2016;14:88-92. [Crossref]
- Brown JG, Folpe AL, Rao P, et al. Primary vascular tumours and tumor-like lesions of the kidney: a clinicopathologic analysis of 25 cases. Am J Surg Pathol 2010;34:942-9. [Crossref] [PubMed]
- Caballes AB, Abelardo AD, Farolan MJ, et al. Pediatric anastomosing hemangioma: case report and review of renal vascular tumors in children. Pediatr Dev Pathol 2019;22:269-75. [Crossref] [PubMed]
- Wetherell DR, Skene A, Manya K, et al. Anastomosing hemangioma of the kidney: a rare morphological variant of hemangioma characteristic of genitourinary tract location. Pathology 2013;45:193-6. [Crossref] [PubMed]
- Zhang W, Wang Q, Liu YL, et al. Anastomosing hemangioma arising from the kidney: a case of slow progression in four years and review of literature. Int J Clin Exp Pathol 2015;8:2208-13. [PubMed]
- Al-Maghrabi HA, Al-Rashed AS. Challenging Pitfalls and Mimickers in Diagnosing Anastomosing Capillary Hemangioma of the Kidney: Case Report and Literature Review. Am J Case Rep 2017;18:255-62. [Crossref] [PubMed]
- Kryvenko ON, Haley SL, Smith SC, et al. Hemangiomas in kidneys with end-stage renal disease: a novel clinicopathological association. Histopathology 2014;65:309-18. [Crossref] [PubMed]
- Memmedoğlu A, Musayev J. Spontaneous rupture of the kidney in the patients with synchronous renal hemangioma and nephrogenic hypertension. Turk J Urol 2015;41:231-4. [Crossref] [PubMed]
- Abboudi H, Tschobotko B, Carr C, et al. Bilateral renal anastomosing hemangiomas: a tale of two kidneys. J Endourol Case Rep 2017;3:176-8. [Crossref] [PubMed]
- Berker NK, Bayram A, Tas S, et al. Comparison of renal anastomosing hemangiomas in end-stage and non-end-stage kidneys: a meta-analysis with a report of 2 Cases. Int J Surg Pathol 2017;25:488-96. [Crossref] [PubMed]
- Mehta V, Ananthanarayanan V, Antic T, et al. Primary benign vascular tumors and tumor-like lesions of the kidney: a clinicopathologic analysis of 15 cases. Virchows Arch 2012;461:669-76. [Crossref] [PubMed]
- Büttner M, Kufer V, Brunner K, et al. Benign mesenchymal tumours and tumor like lesions in end-stage renal disease. Histopathology 2013;62:229-36. [Crossref] [PubMed]
- Cheon PM, Rebello R, Naqvi A, et al. Anastomosing hemangioma of the kidney: radiologic and pathologic distinctions of a kidney cancer mimic. Curr Oncol 2018;25:e220-3. [Crossref] [PubMed]
- Tahir M, Folwell A. Anastomosing hemangioma of the kidney: a rare subtype of vascular tumor of the kidney mimicking angiosarcoma. ANZ J Surg 2016;86:838-9. [Crossref] [PubMed]
- Omiyale AO, Golash A, Mann A, et al. Anastomosing haemangioma of the kidney Involving a segmental branch of the renal vein. Case Rep Surg 2015;2015:927286. [Crossref] [PubMed]
- Chou S, Subramanian V, Lau HMH, et al. Renal anastomosing hemangioma with a diverse morphologic spectrum: report of two cases and review of the literature. Int J Surg Pathol 2014;22:369-73. [Crossref] [PubMed]
- Bean GR, Joseph NM, Gill RM, et al. Recurrent GNAQ mutations in anastomosing hemangiomas. Mod Pathol 2017;30:722-7. [Crossref] [PubMed]
- Bean GR, Joseph NM, Folpe AL, et al. Recurrent GNA14 mutations in anastomosing hemangiomas. Histopathology 2018;73:354-57. [Crossref] [PubMed]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science 2002;296:1636-9. [Crossref] [PubMed]
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med 2010;363:2191-9. [Crossref] [PubMed]
- Urtatiz O, Van Raamsdonk CD. Gnaq and Gna11 in the Endothelin Signalling Pathway and Melanoma. Front Genet 2016;7:59. [Crossref] [PubMed]
- Comi AM, Marchuk DA, Pevsner J. Sturge-Weber Syndrome. In: Rosenberg RN, Pascual JM, editors. Rosenburg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease: Fifth Edition. Elsevier Inc. 2014. p. 945-953
- Joseph NM, Brunt EM, Marginean C, et al. Frequent GNAQ and GNA14 mutations in hepatic small vessel neoplasm. Am J Surg Pathol 2018;42:1201-7. [Crossref] [PubMed]
- Ayturk UM, Couto JA, Hann S, et al. Somatic activating mutations in GNAQ and GNA11 are associated with congenital hemangioma. Am J Hum Genet 2016;98:1271. [Crossref] [PubMed]
- Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med 2013;368:1971-9. [Crossref] [PubMed]
- Liau JY, Lee JC, Tsai JH, et al. High frequency of GNA14, GNAQ, and GNA11 mutations in cherry hemangioma: a histopathological and molecular study of 85 cases indicating GNA14 as the most commonly mutated gene in vascular neoplasms. Mod Pathol 2019;32:1657-65. [Crossref] [PubMed]
- Omiyale AO, Carton J. Clinical and pathological features of primary angiosarcoma of the kidney. Curr Urol Rep 2018;19:4. [Crossref] [PubMed]
- Díaz-Candamio MJ, Pombo F, Lorenzo MJ, et al. Kaposi's sarcoma involving a transplanted kidney: CT findings. AJR Am J Roentgenol 1998;171:1073-4. [Crossref] [PubMed]
- Essid MA, Bouzouita A, Blel A, et al. Masson's tumor of the kidney: a case report. J Med Case Rep 2018;12:376. [Crossref] [PubMed]
- Tran TA, Pernicone P. Anastomosing hemangioma with fatty changes of the genitourinary tract: a lesion mimicking angiomyolipoma. Cent European J Urol 2012;65:40-2. [Crossref] [PubMed]
- Moch H, Humphrey PA, Ulbright TM, et al. (Eds). WHO Classification of Tumours of the Urinary System and Male Genital Organs (4th edition). IARC: Lyon 2016.
Cite this article as: Omiyale AO, Carton J. Clinicopathological and genetic features of anastomosing haemangioma of the kidney. AME Med J 2021;6:30.



