
Etiology–histomorphology–entity correlation in liver pathology: a narrative review
Introduction
Background
The liver as central organ in metabolism is involved in a high number of metabolic processes and is involved in the organ-crosstalk via liver-organ axes. The most important axis is the liver–gut axis, but others come more and more in the focus of interest.
The normal hepatic function is dominated by activities, which are essential for the metabolic and energy homeostasis of the individuum. Most important is the synthesis of glycogen from glucose and other compounds and its breakdown and release into the blood for further utilization by other systems. A high number of biochemical reactions taking place simultaneously in cellular compartments of hepatocytes assisted by other resident and non-resident cells. The reactions include essential steps in anabolism and katabolism of carbohydrates, lipids/lipoproteins, proteins, and specific compounds such as ferriprotoporphyrin IX (haem). In addition, the liver has important abilities for biotransformation and detoxification protecting the whole individuum from potentially harmful substances. The endoplasmic reticulum as well as the mitochondria of hepatocytes are the most important organelles in biotransformation.
The clinical features of liver injuries and disturbances of liver function are highly complex, but a serological set of basic diagnostic screening parameters has been established so far. Using serum bilirubin, gamma-glutamyltransferase, alkaline phosphatase, and aminotransferases a first assessment of liver function is possible. In addition, other parameters including immunological investigations (e.g., antinuclear or antimitochondrial antibodies) and a large number of imaging methods (e.g., ultrasonography, computed tomography, magnetic resonance imaging, and angiography) are routinely used in order to estimate liver function.
Objectives
Despite the high number of clinical and laboratory procedures as well as imaging techniques, the morphological inspection of liver tissues with light microscopy and immunohistochemistry is an essential part contributing to clinical algorithms in the diagnostic procedures and monitor liver diseases. In addition, liver histomorphology is important to stratify and to supervise therapeutic procedures especially in inflammatory and neoplastic diseases.
In the following, an overview about the important cellular players in liver tissues and their histomorphological network in forming pathomorphological patterns is given. In a high number of non-neoplastic liver diseases, mainly of inflammatory or metabolic origin, the morphological features are dominated by an influx of inflammatory cells and/or the occurrence of fibrosis/cirrhosis, and/or lipid accumulation in hepatocytes. These morphological patterns are not always etiology or entity specific and clinical parameters are necessary for further interpretation. In a smaller group of non-neoplastic liver diseases, the morphology of liver tissues is pathognomonic and highly specific for their etiology and entity. In such cases, additional clinical data always promote the histopathological diagnosis (definition of the entity), but they are not essential to identify the etiology of tissue damage. The same is true in hepatocellular tumours and precursors as well as metastases to the liver. The tissue morphology is frequently specific for the entity as well as aspects of the etiology behind and no additional clinical parameter is mandatory for further interpretation. In conclusion, an immediate link between etiology, histomorphology, and entity is possible, but not the rule.
The purpose of the narrative review was to summarize the current knowledge about the correlations of etiology with histomorphology and entity in liver pathology with respect to clinical procedures in diagnostic and treatment of liver diseases. We present the following article in accordance with the Narrative Review reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-21-33/rc).
Methods
Coming from a PubMed search for ‘liver pathology’ with 286,373 results (July 2021), the focus was given to ‘entity’ and ‘etiology’ with 1,772 results in the sort algorithm ‘best match’. Using the additional search term ‘review’ the results was further restricted. Finally, 572 PubMed published articles were identified satisfying the key words: [liver pathology], [entity], [etiology], [review]. The abstract of all these publications were studied and the most suitable articles were selected [criteria for inclusion: (histopathology); for articles before 2020: (cited by more than 9 other articles)]. In addition, there are standard references for well-established routinely used procedures and scoring systems in liver histopathology intending the evaluation of etiology- and entity-related tissue damage. All these articles were selected using the special topic and/or the author(s) and/or the year of publication [e.g., (nonalcoholic steatohepatitis), (Brunt), (Janney), (1999) identifies the paper by Brunt et al., published in 1999 and cited so far by 993 articles]. In order to choose the very best topics and issues to demonstrate etiology-specific aspects of liver pathology, the different causes of liver injury were addressed, i.e., infectious, immune-mediated, metabolic, hepatotoxic mechanical, and genetic, and the most important changes in liver tissue histomorphology were described and characterized with regard to related entities.
Narrative discussion
Normal liver tissue and acting cells
The normal liver tissue consists of a small number of highly differentiated and specialized cell types of epithelial or non-epithelial origin. The most important epithelial cell type and the major parenchymal component is the hepatocyte, that constitute about 80% of the resident cells in the liver. The polyhedral epithelial cell is approximately 35 µm in diameter and arranged in liver-cell plates. There are at least three highly specialized cellular domains, the sinusoidal (basolateral; facing the sinusoid and the perisinusoidal space), the canalicular (which constitutes the bile canaliculus), and the lateral (facing a non-canaliculus part of the intercellular space). In the model of the streaming liver the hepatocytes regenerate and differentiate as they stream from a periportal stem cell compartment to the central vein area in the hepatic lobule (1,2). This is accompanied by an increase in the cell volume as well as a change in the metabolic activities. The definition of hepatic functional units and the hepatic acinus reflects in some aspects this bidirectional relationship of structure and function in the liver. Thus, hepatocytes are exceptionally strongly divided into cellular compartments in terms of their structure and function, which indicate a high polarization (3,4). The hepatocytes are responsible for the system relevant metabolic and energy homeostasis as well as the biotransformation (5).
Hepatocytes are orchestrated by other resident cell types. One of them, the hepatic stellate cell, is found within the space of Disse and is cytomorphological characterized by long cytoplasmic processes as it is typical for pericytes. Stellate cells are not readily visible on light microscopy in HE-stained sections of formalin-fixed and paraffin-embedded (FFPE) liver, but they are detectable by immunohistochemistry using antibodies directed against synaptophysin and other marker proteins. The functional roles attributed to hepatic stellate cells include the synthesis of extracellular matrix proteins, the metabolism of vitamin A, and assistance in liver regeneration.
Another cell type of non-epithelial origin, the Kupffer cell, is found in the lumina of hepatic sinusoids, belong to the mononuclear phagocytic system and acts as a hepatic macrophage. Kupffer cells are more numerous in the periportal zone of sinusoids, which is important for their clearance activities of gut-derived substances, like endotoxin, from the portal blood. Kupffer cells rest on the endothelial lining.
Unlike endothelial cells elsewhere, the sinusoidal endothelial cells are perforated by numerous holes varying in size and diameter. The fenestration is more extensive in the perivenular zone than in the periportal areas and may change in response to several endogenous (e.g., serotonin) or exogenous substances (e.g., ethyl-alcohol). This special endothelial morphology is essential to filter the sinusoidal blood probably assisted by the streaming forces of leucocytes and erythrocytes. Importantly, the normal fenestrated endothelial cells show a number of immunophenotypic differences compared with the non-fenestrated vascular endothelium. The cells are negative for factor VIII, CD34, and GMP140, but do express CD14, CD16, and ICAM-1. Sinusoidal endothelial cells constitute about 10% of the cells in the liver.
The cell types discussed are assisted by other cell types, like liver-associated lymphocytes, and several extracellular compounds. In total, they constitute the liver tissue by the small unit of a central liver cell plate associated with a blood-filled sinus as well as a bile-filled canal of Hering/cholangiole, that initiates the biliary tree. The tree gives the morphological basis for the confluence of bile from the cholangioles through small, interlobular, and septal ducts located in the periphery of portal tracts into the area and segmental ducts.
In the pathomorphological setting, the hallmarks are given with the portal area, the liver cell plates, and the central vein. The portal area consisting of a small developed connective tissue with bile duct, artery segment, venule and few lymphocytes. A clear interface separates hepatocytes, which are arranged in the liver cell plates. The plates are directed to the central vein (Figure 1).
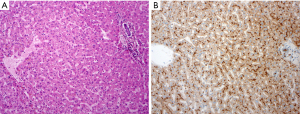
Stereotypic morphological patterns of diseased liver tissue
There are basic morphological features at the histological and cytological level all initiated from a high number of liver injuries clearly differing in their etiology (e.g., infectious, immune-mediated, metabolic, hepatotoxic). These morphologies can be classified in inflammation, injury of living hepatocytes, cell death, fibrosis, regeneration, vascular remodeling, and neoplasia. Importantly, intensity, distribution, and composition of the different features result in stereotypic morphological patterns, which show overlaps concerning their etiology. In particular, the distribution of inflammatory infiltrates, hepatocellular damage, cell loss, fattening and fibrosis within the liver lobe with respect to periportal, mid-zonal and pericentral is important. The histomorphological findings are suitable in different weights to derive safe reliable statements to etiology and entity. An immediate link is possible, but not the rule. Examples are given in the Table 1.
Table 1
| Morphological pattern | Etiology | Entity |
|---|---|---|
| Non-neoplastic: Inflammation, cellular injury (e.g., storage/inclusion of lipids and proteins, cholestasis), cell death, fibrosis/cirrhosis |
Infectious, immune-mediated, metabolic, hepatotoxic (drugs/toxins), mechanical | Echinococcosis, primary biliary cholangitis, Alpha-1 antitrypsin deficiency, hypervitaminosis A, obstructive cholestasis |
| Neoplastic: Atypia, dysplasia, cellular differentiation, vascular remodeling |
Infectious, metabolic, hepatotoxic (drugs/toxins), genetic | Hepatocellular carcinoma, cholangiocarcinoma metastasis |
Frequently, the morphological patterns in diagnostic probes are further modified by the time interval between occurrence of the liver disease and the timepoint of clinical detection and taking liver tissue biopsies. There is often a long-time interval with redundant liver tissue damage and healing, because the strong functional reserve of the liver often masks the liver damage (6,7). Hence, the liver tissues most frequently display changes of ‘chronic liver disease’, which is morphological dominated by fibrosis and chronic inflammation to several degrees. Therefore, the mixed morphological end-stage pattern is not characteristic for its etiology and only a differential diagnosis of several potential causes of the liver disease is possible (Figure 2).
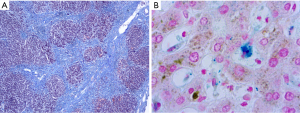
The inflammation pattern is preferentially found in three locations of the liver, the portal tract, the interface between the portal tract and the periportal hepatocytes as well as the lobule. Inflammation as primary cause of liver damage is found in autoimmune hepatitis. The typical histomorphological pattern of autoimmune hepatitis is strong infiltration of portal tracts with CD8+ T-lymphocytes, macrophages, and plasma cells accompanied by periportal interface hepatitis. In hepatotropic viral infection as well as non-alcoholic and alcoholic steatohepatitis the inflammation is an effect of the liver harmful event and aggravates the liver damage.
There are some morphological phenomena indicating severe injury of living hepatocytes. The most important change that is frequently associated with strong liver cell injury is ballooning degeneration of hepatocytes (deranged cellular swelling or oncosis), which could result in cell death. Frequently, lethal and sub-lethal hepatocytes are demarked by inflammatory cells, and accumulation of injury-related proteins like clusterin is found (Figure 3).
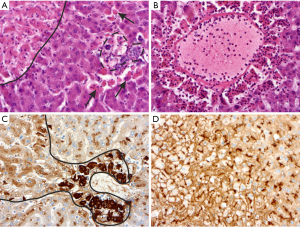
Drug-induced liver injuries comprise a group of histomorphological patterns, which, depending on the pattern intensity, reveal only an inaccurate association with their etiology. This is due to the fact that in addition to an obligate or direct toxic type of injury, there is also an optional or idiosyncratic type as well as various overlap patterns (8). The direct type shows a dose-dependent, reproducible effect, while the idiosyncratic type, with the variants immunological and metabolic-toxic, is neither dose-dependent nor anticipable. The histopathological correlates of drug-induced liver damage are diverse and can be distinguished into a hepatitis type, a bile-draining type, a granulomatous type, a lipid metabolism-associated type, a fibrosing type and a vascular type. The manifestation of such damage patterns depends on the type of the respective drug and the time interval of the drug administration (so-called temporal eligibility). For example, a hepatitis pattern of damage may occur between days and months after administration of isoniazid. Cholangitis or ductopenic patterns are possible up to half a year after administration of amoxicillin/clavulanic acid. The pattern of damage after administration of amiodaron occurring within months may vary between steatohepatitis, phospholipidosis and varying degrees of fibrosis (9). Although numerous data collections and assessment methods exist to enable a rapid correlation of certain pathomorphological patterns of damage of the liver to the etiologically relevant drugs or toxic substances with high precision, the morphological injury pattern per se is often only suitable to narrow the types of substances that may be in question (10). Decisive parameters are the extent of necrosis, the expression of fibrosis, ductopenia and the classification of damage as an acute, subacute or even chronic process.
The total area of all liver sinusoids, seen interface to the hepatocytes as well as their total volume are of utmost importance for the physiological function of the liver. Structural changes in this unit are associated with functional impairments of the liver, but these can only be assessed clinically discretely and insufficiently. For the pathomorphological changes it is crucial that in the sinus the pressure compensation takes place between the systemic and portal venous flow (11). If there are disturbances in these systems, alterations of the sinusoids are often observed, especially enlargement. In chronicity, there is loss of hepatocytes and substitution by connective tissue. The sinusoidal congestion with vascular remodeling, histomorphologically often associated with ectasia of sinus and partly increased expression of CD105, can have very different causes, e.g., chronic right heart failure, hypoxia, increased Kupffer cell activity from hemophagocytosis, non-cirrhotic portal vein hypertension or Budd-Chiari syndrome. The essential morphological facet of hepatic vascular remodeling, the so-called capillarization of sinusoids, goes hand in hand with the formation of a basal membrane and processes of neoangiogenesis with expression of CD34. This can be observed especially in the expansion of anti-CD34 stained cells in zone 1 of the liver lobule (12). When the changes are long-standing, a fibrotic-tissue conversion with parenchyma replacement may occur. In the course of remodeling, heterogeneous blood circulation and blood flow can also result in a non-fibrotic small-nodular conversion of liver tissue, called nodular regenerative hyperplasia. It is assumed that liver areas with reduced perfusion are to become locally atrophic, while in the immediate vicinity a compensatory hypertrophy can be found with normal or slightly increased perfusion. Molecularly, an inactivation of the Notch signal transduction as well as cellular aging may be of importance (13,14).
Other morphologically defined forms of sinusoidal circulation disorders are the peliosis hepatis, which is characterized by differently pronounced cystic cavities with defective or missing reticulo-lamellar boundaries, and the sinusoidal remodeling in cirrhosis of the liver. Again, these are stereotypical histomorphological findings, but they have many different etiologies. Thus, in particular, drugs can induce findings of a microscopic peliosis hepatis, while various steroids are etiologically relevant for already macroscopic detectable forms of peliosis (15).
Another important histomorphological reaction pattern of the liver is the storage of lipids, which can be found particularly pronounced in the hepatocytes and also in the hepatic stellate cells. In order to quantify the fattening of hepatocytes histomorphologically, threshold values were defined (16). The lower limit is a fattening of a maximum of 5% of hepatocytes, while from 50% the terms steatosis hepatis or fatty liver disease (FLD) are used. With regard to the pattern of fattening, a macrovesicular is distinguished from a microvesicular form. As a measure of delimitation, the size of the fat vacuole is often related to the size of the nucleus of the affected hepatocytes. In the macrovesicular fattening, the fat vacuoles are larger than the nucleus, while in microvesicular fattening they are smaller. Typically, the nucleus is largely left in its configuration and intracellular localization during microvesicular fattening, while macrovesicular fattening marginalizes the nucleus and sometimes impressions of the nuclear membrane can be observed. Mixed forms of hepatocellular fattening are comparatively common and can also occur alternately with the pure fattening patterns.
The semiquantitative detection of the degree of fattening of hepatocytes is in discussion. Thus, the view is preferred to refer the percentage of fattening to the total number of hepatocytes (17). However, there is also the procedure to refer the area of the fattening to the total area of the liver parenchyma under investigation. In principle, a distinction of the degree of fattening can be given with mild (5–32%), moderate (33–66%) and high (>66%).
In an etiology-specific view it must be distinguished the stereotypical pattern of secondary fat retention in liver epithelial cells, e.g., drug- or virus hepatitis-associated, from the alcoholic fatty liver disease (AFLD) and non-alcoholic fatty liver disease (NAFLD) (18). Drugs that are more likely to develop FLD are methotrexate, corticosteroids, as well as valproic acid and tetracyclines. In some cases, fattening may be associated with an inflammatory component and signs of increased damage to the liver epithelium as ballooning. In these cases, where an inflammatory component is found, the histomorphological pattern should be addressed as steatohepatitis. Importantly, morphological changes like megamitochondria, nuclear glycogen inclusions, and apoptotic hepatocellular death are frequently found in hepatocellular fattening, but they are non-characteristic concerning their etiology.
In order to assess the stereotypical patterns regarding the extent of clinically relevant liver damage even without direct knowledge of etiology, the quantifiable assessment of inflammation, fibrosis, fattening and necrosis has been proven. It has been shown that a four-stage classification of both the extent of inflammation (degree of inflammation) and the extent of fibrosis (stage of fibrosis) is useful for routine feasibility and reproducibility. This approach is largely based on the Desmet classification, which was originally developed for the classification of liver changes in hepatitis C (19). However, it has been shown that for the semi-quantifiable assessment of liver tissues, this classification principle is also appropriate if it is not a viral hepatitis etiology. The use of this score in particular in liver damage that is not induced by hepatotropic viruses therefore seems plausible and justified. This approach should be excluded in routine pathology from liver tissues which indicate increased fattening (>20% of hepatocytes) or otherwise evidence of the presence of FLD (e.g., clinical context). For the evaluation of such fatty liver tissues with regard to the expression of fattening, inflammation/liver epithelial damage and fibrosis, scoring systems have prevailed, which allow a more optimal semiquantitative assessment of the changes. The approach developed by Brown and Kleiner is most prevalent in this regard (20). In this scoring system, the fibrosis that initially manifests itself in the center of the liver lobule and the demarcation of inflammatory foci with so-called ballooning of the hepatocytes is taken into account (Table 2).
Table 2
| Grading | Staging | Steatosis/steatohepatitis | Staging in steatosis/steatohepatitis |
|---|---|---|---|
| 0: No inflammation | 0: No fibrosis | Fattening: <5% (Score 0) 5–33% (Score 1) >33–66% (Score 2) >66% (Score 3) |
0: No fibrosis |
| 1: Minimal (portal inflammation) | 1: Mild (portal fibrosis) | 1A: Minimal (perisinusoidal fibrosis; zone 3) 1B: Mild (perisinusoidal fibrosis; zone 3) 1C: Minimal/Mild (portal/periportal) |
|
| 2: Mild (portal inflammation, initial interface hepatitis) | 2: Moderate (portal fibrosis, in-/complete septa) | ||
| 3: Moderate (portal inflammation, interface hepatitis, single cell necrosis) | 3: Severe (portal fibrosis, complete septa with architectural damage) | Inflammation: No (Score 0) <2 foci /MPF (Score 1) 2–4 foci /MPF (Score 2) >4 foci /MPF (Score 3) |
2: Moderate (perisinusoidal and portal/periportal fibrosis) |
| 4: Severe (portal inflammation, interface hepatitis, increased cell necrosis, panlobular necrosis) | 4: Cirrhosis (complete architectural fibrotic damage) | Ballooning: No (Score 0) Few ballooning (Score 1) Ballooning (Score 2) |
3: Severe (fibrosis with complete septa and initial architectural damage) |
| Scoring: 0–2: no steatohepatitis 3–4: borderline ≥5: steatohepatitis |
4: Cirrhosis (complete architectural fibrotic damage) |
The inflammatory changes in infection with hepatotropic viruses depend in their form on the immune competence of the infected individual and the primary viral load. Although histomorphologically distinct changes are found that speak for the presence of a specific virus type (e.g., focal fattening in hepatitis C), the diagnosis of the virus type from histomorphology is problematic and significantly inferior to serology. In addition, there are drug-induced liver injuries with an inflammatory histomorphological pattern, the morphological demarcation of both viral hepatitis and autoimmune hepatitis is almost impossible. Only through the use of additional examinations, e.g., tissue immunostainings, can the conventional light microscopic patterns be further resolved in a etiologically relevant manner.
Similar to the histomorphological variability of inflammatory patterns of liver tissue in viral hepatitis, there is also a spectrum of morphological findings in autoimmune hepatitis depending on the stage or course and type of the disease. Currently, three different types are distinguished, which diversify in terms of the autoantibodies to be observed, the age of manifestation and the clinical course. The fulminant course is associated with a pronounced inflammatory loss of the liver parenchyma (acute liver dystrophy), while a pan-acinar inflammatory image is seen in the acute-onset autoimmune hepatitis. Especially with dominance of centro-lobular necrosis, the demarcation of drug-induced hepatitis-like intolerance reactions without further clinical data is almost impossible (21). The histomorphological multifaceted richness of chronic autoimmune hepatitis and its acute relapses is considerable and therefore not necessarily etiology-specific. The inflammatory component is typically admixed with parenchyma fibrosis. In the foreground of the inflammatory findings is the so-called interface hepatitis. It is located in the boundary area of portal field and liver cell plates. Typically, the inflammatory infiltrate includes lymphocytes and plasma cells colocalized with swelling and pyknosis of the periportal hepatocytes. Here, too, there is a high degree of diversification, so plasmacellular infiltrates can be completely absent. Clusters of at least 5 plasma cells (in particular with dominance of IgG) are found in portal fields in courses that are histomorphologically more typical of autoimmune hepatitis, which should also give a certain distinction to active hepatitis C (22). Other findings may include hepatocellular syncytial giant cells, periportal liver cell rosettes and hepatocellular emperipolesis. Possibly due to the blurring of morphological findings regarding autoimmune hepatitis etiology, there is no independent evaluation system for grading and staging of autoimmune hepatitis so far. In this respect, the index systems according to Desmet or Ishak are often used here (23) and contribute to the clinical scoring (24). In the multicenter study by Hennes several hundreds of probes were investigated and strong evidence was found that a reliable diagnosis of autoimmune hepatitis can be made by scoring of autoantibodies, immunoglobulin G, histology, and exclusion of viral hepatitis (24).
A histological finding, which is also uncharacteristic with regard to its etiology, is the loss of bile ducts (ductopenia/vanishing bile duct syndromes). In these cases, the portal field matrix shows only vascular structures, but no bile ducts. The verification of such changes can be problematic, since also in the normal case a part of the portal fields is represented without a bile duct. A certain basic rule here is that about 10% of portal fields without bile ducts are normal (0.9–1.8 ducts per portal tract). However, if more than 50% of the portal fields are without bile ducts (<0.5 bile ducts per tract; at least 10 portal tracts under observation), a manifest ductopenia can be assumed (25). Etiologically relevant for ductopenia/vanishing bile duct syndromes may be in particular autoimmune cholangitis, graft-versus-host disease, ischemic cholangitis and the large group of drug or toxin-induced bile duct damage (e.g., induced by carbamazepine or amoxicillin–clavulanic acid). It is important to distinguish the hereditary/syndromic diseases of childhood (e.g., Alagille syndrome). For the safe demarcation and classification with regard to the histomorphological findings, the clinical context is again decisive.
Etiology- and entity-specific morphological patterns of diseased liver tissue
AFLD and NAFLD
As already mentioned above, etiology-specific pattern can be distinguished with regard to liver fattening. The two outstanding diseases in this regard are the AFLD and the NAFLD. Concerning their histological patterns, both diseases show clear overlaps. Thus, the safe morphological demarcation without knowledge of the clinical constellation is difficult. The appearance or absence of Mallory bodies is not necessarily evidence of the presence of an AFLD or NAFLD (26). However, the pattern of fibrosis is helpful to separate AFLD from NAFLD in histomorphology. In addition, the extent of fattening is often more pronounced in NASH than in ASH, which also shows increased microvesicular patterns. ASH, but not NASH, is also associated with cholestasis/bilirubinostasis in about 30% of cases. Moreover, the veno-occlusive changes with sclerosing hyaline necrosis are pointing away for the ASH.
For the pathogenesis of NAFLD, the disproportion of the availability of free fatty acids and reduced beta-oxidation is important. The storage of lipids in the cytoplasm of hepatocytes is also promoted by insulin resistance, which indicates the presence of metabolic syndrome. The extent to which the composition of the intestinal microbiome through liver-gut axis can influence the manifestation of NAFLD is currently the subject of intensive research. The transition of fattening into steatohepatitis is triggered by both direct and indirect lipotoxicity on the hepatocytes. An intense damage mechanism is the oxidative stress.
Histologically, a rather macrovesicular type of fattening can be found in the early stages of NAFLD in the center of the liver lobe (Zone 3). In advanced stages of the disease, panlobular fattening is seen, which often shows microvesicular patterns in addition to macrovesicular fattening. In addition to the fattening of hepatocytes, there are focal inflammatory infiltrates within the liver lobules, which predominant include T-lymphocytes. In contrast to lymphocytes, neutrophils are comparatively rare. However, they are often found in the neighborhood of Mallory bodies. The more the number of neutrophils increases, the sooner the presence of viral hepatitis should be included in the differential diagnosis. In the steatohepatitic setting, so-called lipogranulomas are increasingly configuring. They consist of inflammatory cells rosetting a fattened hepatocyte. The typical morphological correlate of hepatocellular damage in steatosis/steatohepatitis is the ballooning of hepatocytes. Affected hepatocytes show a brightening of the cytoplasm with significant volume increase, rounding off the cell contour and nuclear enlargement with pronounced demarcation of a nucleolus (20). It should be noted once again that the discussed patterns of NASH depend on the stage of the disease. Finally, another important variable that influences the histological appearance of NASH should be taken into account; this is the patient’s age. In pediatrics, the histomorphological NASH findings differ significantly from the changes described above. Thus, fattening of hepatocytes, inflammation and fibrosis are more likely to be observed periportal in pediatric NASH. In addition, the ballooning of hepatocytes is seldom and the configuration of Mallory bodies is a rarity.
Alpha-1 antitrypsin deficiency
Alpha-1 antitrypsin deficiency belongs to the group of endoplasmic storage diseases. In such diseases, a molecular abnormality leads to misfolding of secreted proteins, like alpha-1 antitrypsin or fibrinogen, that disturbs the protein transport within the endoplasmic reticulum and consequent accumulation in the hepatocellular cytoplasm (27).
Alpha-1 antitrypsin is a plasma glycoprotein which is mainly synthesized by hepatocytes. The protein, which is also synthesized by other tissues than the liver, is a competitive inhibitor of leucocyte elastase. Morphologically, the hallmark of the disease, especially the Z-type of alpha-1 antitrypsin deficiency, are diastase-resistant PAS-positive globules in hepatocytes (Figure 4). They represent the retention of the abnormal, non-functional and unsecretable protein within the rough endoplasmic reticulum. The globules are subsequently found in livers of individuals homozygous or heterozygous for alpha-1 antitrypsin deficiency other than the Z-type. In early stages the inclusions appear as crescents or rectilinear structures in the cytoplasm of hepatocytes. The influx of inflammatory cells and hepatitis are able to aggravate the disease progression with ductular reaction, bridging fibrosis and development of cirrhosis. Sometimes paucity of intrahepatic bile ducts is found and features of chronic cholatestasis and copper storage are seen.
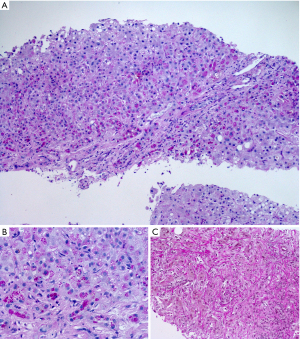
Echinococcosis
Certain infectious diseases are associated with very characteristic changes in the liver, which allow the etiologically correct classification even without knowledge of further clinical data and connections. A typical example of this is infection with cestodes. Human pathogenically relevant in this context is in particular the echinococcosis. This is the infection by the larval forms of Echinococcus tapeworms. The most common is Echinococcus granulosus, less common Echinococcus multilocularis. Intermediate hosts are usually rodents, but many mammals can serve including humans.
Unilocular hydatidosis is the classic morphological pattern caused by Echinococcus granulosus. After ingestion, the eggs hatch and the larval oncospheres are drained into the liver by the portal vein. Frequently, the right lobe of the liver is affected by the growth of a typical hydatid cyst. The cyst may be single and unilocular or contain several daughter cysts which have established by invagination of the germinal membrane. The structural components of the hydatid cyst wall are very characteristic. There is an outer acellular membrane of about 0.1 cm width. This membrane is in contact with the germinal layer, a nucleated lining. From the germinal membrane budding of protoscolices is found. These are ovoid or round particles with circles of hooklets (Figure 5). The unilocular hydatidosis is found with two typical patterns of histology. In the first one, there is the vital cyst with nucleated germinal membrane and protoscolices. The other one is dominated by wholly or partly degenerated collapsed cysts forming closely rolled PAS positive and anucleate membranes devoid of viable protoscolices. There is only a weak immune reaction of the host with few granulation tissue and thin fibrosis adjacent to vital or degenerative cysts.
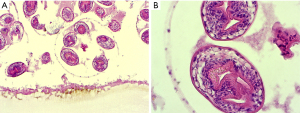
Echinococcus multilocularis (alveolaris) causes a more aggressive disease, but is less common. In the liver, there are irregular multilocular cysts filled with necrotic material. In contrast to the cysts of Echinococcus granulosus the PAS positive laminated membrane is often fragmented and without any nucleated germinal membrane. Importantly, protoscolices are not seen in human infection. The fragmented membranes are able to disseminate in the necrotic tissues accompanied by a variable host response with granulomatous reaction with granulocytes.
Primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC)
PBC (formally primary biliary cirrhosis) and PSC are inflammatory liver diseases both probably autoimmune triggered. At least in certain stages of these diseases, pathognomonic histomorphological patterns of liver tissues were found. In particular, the histomorphological findings of the exclusively intrahepatic manifest PBC show stage-like differences. In the early stage of the disease, inflammation is concentrated on the portal field. It is lymphocytic-dominated with addition of plasma cells (mostly IgM +), manifestation of lymphoepithelial lesions with destruction of the bile duct epithelium and occasional formation of bile duct-related granulomas. Typically, neutrophilic granulocytes are in the minority. While in the early stages of PBC the number of affected portal fields can still be small, with the progression of the disease to stage 2 an increasing number of portal fields are affected and the inflammatory cell infiltrate crosses the portal field boundaries. In stage 2, biliary interface hepatitis occurs with concomitant neo-ductular proliferates, periductular fibrosis and cholestasis. The congestion of bile acids manifests itself in the hepatocytes as feathery degeneration and there is an increasing retention of copper-associated proteins. In stages 3 and 4, the fibrosing reaction shows more and more dominance over inflammation and there is a significant reduction in the number of bile ducts. In stage 4, ductopenia and cholestasis are found in the setting of biliary cirrhosis (28,29).
The PSC is characterized as an immunomediated disease of intrahepatic and/or extrahepatic bile ducts. It dominates a cholestatic image with increasing connective tissue organization of the bile ducts and parenchyme fibrosis up to cirrhosis of the liver. Histologically, fibrosis established around bile ducts with concomitant edema, lymphoplasmic infiltration and interface activity is found. The epithelium of the bile ducts shows degenerative changes with apoptosis and areas of biliary metaplasia of the associated hepatocytes. The small interlobular bile ducts typically have a widening of their basal membrane in the PAS staining. It is characteristic of the PSC that it manifests itself heterogenous in liver tissue and differs in its progress. Due to this phenomenon, different histomorphological stages of the disease are often present side by side. Special forms of this cholangiopathy include the PSC overlap syndrome with autoimmune hepatitis and the sclerosing cholangitis with granulocytic epithelial lesions. Sclerosing cholangitis with granulocytic epithelial lesions was addressed in a well-designed retrospective study including liver biopsies of 103 children suffering from autoimmune sclerosing cholangitis and 142 adults with PSC (30). Neutrophilic bile duct injury was found in 4% of autoimmune sclerosing cholangitis and in 0.7% of PSC. The autoimmune hepatitis overlap was also described for the PBC. The PBC overlap with PSC is a rarity (31).
Neoplastic diseases
Neoplastic diseases of the liver comprise a wider range of various tumor diseases. Large groups can be distinguished by liver-own (primary) and liver-metastatic (secondary) tumors as well as carcinoma with unknown primary (CUP) of the liver. Their common feature is the autonomous proliferation of cells largely outside of immune regulation. Although the etiology of the various tumor types can often be multifactorial and ultimately unclear, direct therapeutic consequences for the clinical field derives from the respective morphologically, immunophenotypically and partly molecularly defined entities. In particular, the most important primary liver carcinomas are hepatocellular carcinoma (HCC) and cholangiocarcinoma of the small or large duct type.
HCC
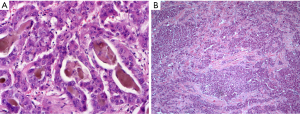
HCC is the classic liver-own neoplasia, which is due in its origin to hepatocytes (profile of immunostainings: HepPar1 +, arginase +, CD10 +, pCEA +, keratin 8 +, keratin 18 +, keratin 7 ‒/+). Following the recent WHO proposal for the classification of HCCs at least eight subtypes could be separated by histological and molecular characteristics. Examples are given in Figure 6. In the pathogenesis of HCCs histomorphologically distinct progenitor lesions, the so-called dysplastic foci, are found. These lesions consist of a clonal expansion of hepatocytes with an extent of less than 0.1 cm. Lesions larger than 0.1 cm are called dysplastic nodules and are subdivided into low-grade and high-grade dysplastic nodules in terms of the expression of dysplasia and additive architectural disorders (e.g., pseudoglandular patterns, size of liver plates more than two cell layers). Vascular aberrations can already be found in low-grade nodules (32). Due to the micro-focal occurrence of an interstitial and/or vascular invasion, the HCC is distinguished from the dysplastic nodule. By definition, the term ‘small HCC’ is used for an invasive liver cell neoplasia with a size less than 2 cm. Importantly, HCCs with early (i.e., vague nodular HCC) as well as advanced tumor-biology (i.e., distinct nodular HCC) are subsumed under this term. Starting from such small lesions, histologically and cytologically evident HCCs develop through progressive hepatocellular dedifferentiation. For the diagnostic classification of a neoplastic lesion as HCC, the immunohistological detection of glypican 3, HSP70 (or 14-3-3sigma) and glutamine synthetase has proven itself (33). The marker profile was further established in the complex experimental study by Reis, where the biomarker candidates were identified using comparative proteomics of HCCs and corresponding non-tumorous liver tissues (n=14) (34). With high specificity and medium to good sensitivity, the positive detection of two of the three markers glypican 3, HSP70 (or 14-3-3sigma) and glutamine synthetase indicates the presence of an HCC. Especially in early HCCs, however, there are aberrant patterns of immunostainings. Thus, glypican 3 may be missing and CD34 can show an inconspicuous distribution pattern in view of a dominant portal venous blood supply without complete diffuse capillarization of the sinusoids.
Summary
The morphological features of liver injuries and disturbances in liver function are complex, but a basal spectrum of stereotypical histomorphological and cytomorphological patterns are found. They include fibrosis, inflammation, fattening, storage of protein-rich materials, cell death and neoplastic transformation. The variability in these morphological categories, assessed with a set of scoring systems, predicts the link between etiology and entity. However, there are histomorphological and cytomorphological constellations, in particular inflammatory lesions of the liver, which need the clinical context for final interpretation.
Multidisciplinary collaboration will be essential to further improve the diagnostic power of the etiology–histomorphology–entity correlation. In particular, identification and establishment of additional biomarkers to measure and visualize the dynamic of the three highly specialized hepatocellular domains as well as the streaming of hepatocytes in inflammatory disorders are essential. The read-out of such parameters should be more and more assisted by algorithms of artificial intelligence.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ralf Weiskirchen and Wolfgang Stremmel) for the series “Liver Diseases: Symptoms, Causes and Novel Therapies” published in AME Medical Journal. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-21-33/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-21-33/coif). The series “Liver Diseases: Symptoms, Causes and Novel Therapies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lukacs-Kornek V, Lammert F. The progenitor cell dilemma: Cellular and functional heterogeneity in assistance or escalation of liver injury. J Hepatol 2017;66:619-30. [Crossref] [PubMed]
- Russell JO, Monga SP. Wnt/β-Catenin Signaling in Liver Development, Homeostasis, and Pathobiology. Annu Rev Pathol 2018;13:351-78. [Crossref] [PubMed]
- Müsch A. The unique polarity phenotype of hepatocytes. Exp Cell Res 2014;328:276-83. [Crossref] [PubMed]
- Gissen P, Arias IM. Structural and functional hepatocyte polarity and liver disease. J Hepatol 2015;63:1023-37. [Crossref] [PubMed]
- Boyer JL. Bile formation and secretion. Compr Physiol 2013;3:1035-78. [Crossref] [PubMed]
- Sakka SG. Assessing liver function. Curr Opin Crit Care 2007;13:207-14. [Crossref] [PubMed]
- Horvatits T, Drolz A, Trauner M, et al. Liver Injury and Failure in Critical Illness. Hepatology 2019;70:2204-15. [Crossref] [PubMed]
- Watkins PB, Seeff LB. Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology 2006;43:618-31. [Crossref] [PubMed]
- Torous VF, De La Cruz AL, Naini BV, et al. Cholangitis Lenta: A Clinicopathologic Study of 28 Cases. Am J Surg Pathol 2017;41:1607-17. [Crossref] [PubMed]
- Danan G, Teschke R. Drug-Induced Liver Injury: Why is the Roussel Uclaf Causality Assessment Method (RUCAM) Still Used 25 Years After Its Launch? Drug Saf 2018;41:735-43. [Crossref] [PubMed]
- Brunt EM, Gouw AS, Hubscher SG, et al. Pathology of the liver sinusoids. Histopathology 2014;64:907-20. [Crossref] [PubMed]
- Lalor PF, Lai WK, Curbishley SM, et al. Human hepatic sinusoidal endothelial cells can be distinguished by expression of phenotypic markers related to their specialised functions in vivo. World J Gastroenterol 2006;12:5429-39. [Crossref] [PubMed]
- McLean AJ, Cogger VC, Chong GC, et al. Age-related pseudocapillarization of the human liver. J Pathol 2003;200:112-7. [Crossref] [PubMed]
- Reshamwala PA, Kleiner DE, Heller T. Nodular regenerative hyperplasia: not all nodules are created equal. Hepatology 2006;44:7-14. [Crossref] [PubMed]
- Hayward SR, Lucas CE, Ledgerwood AM. Recurrent spontaneous intrahepatic hemorrhage from peliosis hepatis. Arch Surg 1991;126:782-3. [Crossref] [PubMed]
- Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 1999;94:2467-74. [Crossref] [PubMed]
- Bedossa P, Poitou C, Veyrie N, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology 2012;56:1751-9. [Crossref] [PubMed]
- Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263-73. [Crossref] [PubMed]
- Desmet VJ, Gerber M, Hoofnagle JH, et al. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 1994;19:1513-20. [Crossref] [PubMed]
- Brown GT, Kleiner DE. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism 2016;65:1080-6. [Crossref] [PubMed]
- Tiniakos DG, Brain JG, Bury YA. Role of Histopathology in Autoimmune Hepatitis. Dig Dis 2015;33:53-64. [Crossref] [PubMed]
- Gurung A, Assis DN, McCarty TR, et al. Histologic features of autoimmune hepatitis: a critical appraisal. Hum Pathol 2018;82:51-60. [Crossref] [PubMed]
- Strassburg CP, Beckebaum S, Geier A, et al. S2k Leitlinie Autoimmune Lebererkankungen. Z gastroenterol 2018;55:1135-226.
- Hennes EM, Zeniya M, Czaja AJ, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008;48:169-76. [Crossref] [PubMed]
- Ludwig J, Wiesner RH, LaRusso NF. Idiopathic adulthood ductopenia. A cause of chronic cholestatic liver disease and biliary cirrhosis. J Hepatol 1988;7:193-9. [Crossref] [PubMed]
- Scaglioni F, Ciccia S, Marino M, et al. ASH and NASH. Dig Dis 2011;29:202-10. [Crossref] [PubMed]
- Callea F, de Vos R, Togni R, et al. Fibrinogen inclusions in liver cells: a new type of ground-glass hepatocyte. Immune light and electron microscopic characterization. Histopathology 1986;10:65-73. [Crossref] [PubMed]
- Scheuer P. Primary biliary cirrhosis. Proc R Soc Med 1967;60:1257-60. [Crossref] [PubMed]
- Nakanuma Y, Zen Y, Harada K, et al. Application of a new histological staging and grading system for primary biliary cirrhosis to liver biopsy specimens: Interobserver agreement. Pathol Int 2010;60:167-74. [Crossref] [PubMed]
- Zen Y, Grammatikopoulos T, Heneghan MA, et al. Sclerosing cholangitis with granulocytic epithelial lesion: a benign form of sclerosing cholangiopathy. Am J Surg Pathol 2012;36:1555-61. [Crossref] [PubMed]
- Boberg KM, Chapman RW, Hirschfield GM, et al. Overlap syndromes: the International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol 2011;54:374-85. [Crossref] [PubMed]
- Park YN, Yang CP, Fernandez GJ, et al. Neoangiogenesis and sinusoidal "capillarization" in dysplastic nodules of the liver. Am J Surg Pathol 1998;22:656-62. [Crossref] [PubMed]
- Liu CC, Jan YJ, Ko BS, et al. 14-3-3σ induces heat shock protein 70 expression in hepatocellular carcinoma. BMC Cancer 2014;14:425. [Crossref] [PubMed]
- Reis H, Pütter C, Megger DA, et al. A structured proteomic approach identifies 14-3-3Sigma as a novel and reliable protein biomarker in panel based differential diagnostics of liver tumors. Biochim Biophys Acta 2015;1854:641-50. [Crossref] [PubMed]
Cite this article as: Gassler N, Press A, Rauchfuß F, Theis B, Kaemmerer E. Etiology–histomorphology–entity correlation in liver pathology: a narrative review. AME Med J 2022;7:14.

