
Mediastinal restaging with transcervical extended mediastinal lymphadenectomy in patients with locally advanced non-small cell lung cancer treated with pneumonectomy
Introduction
Non-small cell lung cancer (NSCLC) is diagnosed as locally advanced disease in around 30% of patients. This group comprises a wide spectrum of clinical presentations with generally poor prognosis, often with metastatic mediastinal lymph nodes (LNs) and/or centrally located tumour requiring pneumonectomy for complete resection. Optimal treatment of these patients remains controversial. The usual approach is a multimodal treatment including platinum-based chemotherapy with some form of local treatment, like surgery, radiotherapy or both. Due to reported excessive perioperative mortality associated with pneumonectomy after neoadjuvant therapy, this form of treatment is avoided in patients requiring multimodal approach in most centers (1). On the other side, there are reports of good short-term and long-term outcomes after pneumonectomy performed as part of the multimodal approach (2). While persistence of the mediastinal LN metastases after induction treatment is associated with poor prognosis, outcome after surgical treatment may be good in patients with mediastinal nodal downstaging operated in experienced centers (3). Thus, accurate mediastinal restaging after neoadjuvant therapy is crucial for appropriate selection of treatment for patients, especially candidates for pneumonectomy. However, optimal method of the mediastinal restaging is still a matter of a debate. As diagnostic yield of imaging techniques (CT, PET/CT) is low, accurate determination of the mediastinal LN status requires some form of tissue sampling (4,5). Endoscopic methods like transesophageal ultrasound-guided fine needle aspiration (EUS-FNA) or endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) allow for the cytologic examination of the samples of the mediastinal LNs. However, negative results of endoscopic biopsies should be confirmed by surgical staging, due to low negative predictive value of the endoscopic methods (6).
Our team introduced transcervical extended mediastinal lymphadenectomy (TEMLA) in 2004 (7). It is a surgical inspection of the mediastinum with high diagnostic yield for detecting metastases in the mediastinal LNs. Here we report long-term outcomes of pneumonectomy in the management of patients with locally advanced NSCLC after neoadjuvant therapy, selected for surgical treatment on the basis of negative results of the restaging of the mediastinum with TEMLA. We present the following article in accordance with the STROBE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-21-38/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of our hospital (No. 1/2021) and individual consent for this retrospective analysis was waived. Hospital medical charts of all patients with locally advanced NSCLC (excluding patients with N3 disease) who underwent mediastinal restaging with TEMLA after induction chemotherapy or chemoradiotherapy from 1st January 2007 to 31st December 2015 at the Department of Thoracic Surgery, Pulmonary Hospital, Poland, was retrospectively analyzed. A total of 169 patients were diagnosed as metastases-free in the mediastinal LNs after restaging with TEMLA and underwent subsequent thoracotomy with pulmonary resection. Within this group, there were 68 patients who underwent pneumonectomy and who are the subjects of this study. After completion of induction treatment, all patients underwent CT and EBUS/EUS. Patients with stable disease or remission on CT and with negative results of endoscopic mediastinal restaging underwent final restaging with TEMLA. Only those with negative TEMLA proceed to pneumonectomy. The number of operated cases during the study period determined the sample size. There were 52 men and 16 women with age ranging from 47 to 75 years (mean 59.4 years). There were 53 squamous cell carcinomas, 11 adenocarcinomas, 1 mixed carcinoma (adenocarcinoma-squamous cell) and 3 non-small cell lung carcinomas. Neoadjuvant chemotherapy with platinum doublets was given to 47 patients and neoadjuvant chemoradiotherapy to 21 patients. Metastases to the mediastinal LNs (N2) before induction treatment were cytologically confirmed in 26 patients (38.2%) and in 6 patients (8.8%) N2 disease was diagnosed on the basis of PET/CT or chest CT scans showing enlarged or active mediastinal LNs. Thirty-six patients (53%) underwent induction therapy to downsize centrally located neoplasms with borderline resectability, without evidence of the mediastinal LNs involvement. Before induction treatment, 14 tumours were clinically staged as IIA, 16 as IIB, 36 as IIIA and 2 as IIIB. After induction, 15 tumours were clinically restaged as IB, 26 as IIA, 22 as IIB and 5 as IIIA. All patients were seen in follow-up for at least 60 months after pneumonectomy or until death.
Surgical technique of TEMLA was described elsewhere (7). Collar incision in the neck was used and the sternal manubrium was elevated with a retractor. Radical dissection of the mediastinal nodal stations 1, 2R, 2L, 4R, 4L, 7 and upper 8 was performed. Parts of the procedure involving dissection of LN stations 4R, 4L, 7 and 8 were performed in the mediastinoscopy-assisted technique. In case of left-sided tumours, nodal groups 5 and 6 were also dissected, usually with the aid of videothoracoscope inserted through the neck incision. Pneumonectomy was performed in standard fashion through anterolateral, muscle-sparing thoracotomy. Main bronchus stump closure was performed using technique described by Asamura et al. (8). Bronchial stump was not routinely covered. All pulmonary resections included completion mediastinal LN dissection.
Statistical analysis
8th edition of American Joint Commission on Cancer Classification was used to stage the tumours (9). Overall survival was defined as the time from pneumonectomy to death from any cause. Survival rates was calculated using Kaplan-Meier method and compared with log-rank test. Statistical significance was defined as P<0.05. All data analyses were performed using Prism 5 (GraphPad Software Inc.).
Results
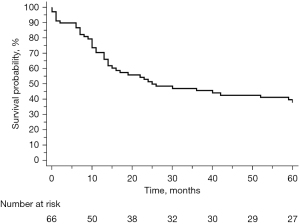
There were no complications after TEMLA. After pneumonectomy, persistent metastatic N2 nodes were found in 4 patients (5.9%) and positive N1 nodes in 13 patients (19.1%). Complete pathological response to the induction treatment was found in 11 patients (16.2%, 10 patients underwent chemoradiotherapy). There were 32 right and 36 left pneumonectomies. R0 resection was achieved in 66 patients (97%), while 2 patients had R1 resection. Overall 30-day and 90-day mortality was 2.9% and 10.3%, respectively. There were 6 bronchial fistulas (4 after right pneumonectomy, 2 after left pneumonectomy). All patients with bronchial stump insufficiency underwent surgical treatment (thoracostomy, myoplasty), 5 were cured and 1 patient died. Median follow-up was 25 months. Forty-one patients died during the follow-up period and overall 5-year survival of patients who underwent pneumonectomy after negative TEMLA was 39.7% (including false negative results, Figure 1). The 5-year survival was similar in patients who underwent right and left pneumonectomy (40.6% vs. 38.9%, P=0.66, Figure 2). Overall 5-year survival was similar in patients who had mediastinal nodal metastases diagnosed before treatment and in patients who were not diagnosed with N2 disease (44.4% vs. 34.4%, P=0.67, Figure 3). Overall 5-year survival was not statistically different in patients with true mediastinal LN clearance and in patients with persistent N2 nodes and falsely negative TEMLA results (39.7% vs. 25%, P=0.43, Figure 4).
Discussion
In this study, we showed that pneumonectomy after neoadjuvant treatment may lead to good long-term outcomes in NSCLC patients with mediastinal LN downstaging when they are precisely selected for surgery on the basis of invasive mediastinal restaging with TEMLA. Optimal treatment of patients with locally advanced NCSLC remains controversial. Those patients benefit from multimodal approach, with induction chemotherapy of chemoradiotherapy followed by surgery. Induction therapy is employed to downsize locally advanced tumours and render them resectable. It may also eradicate metastases to mediastinal LN and systemic micrometastases. However, both neoadjuvant chemotherapy and radiotherapy were shown to increase perioperative morbidity and mortality (10,11). Especially pneumonectomy performed in patients with mediastinal LN metastases who underwent neoadjuvant treatment was reported to negatively affect survival (12). Thus, many authors recommend to avoid pneumonectomy, limiting treatment of patients with locally advanced NSCLC to radical chemoradiotherapy (1,13,14). However, such approach seems to be associated with suboptimal long term survival (15). On the other hand, there are reports of acceptable safety and favorable outcomes of pneumonectomy in the setting of multimodal approach (2,16). In a seminal paper by Weder et al., 176 stage III NSCLC patients underwent pneumonectomy after chemotherapy or chemoradiotherapy, with excellent 38% 5-year survival and impressive, 3% perioperative mortality. Similar long-term survival was also reported by other groups (17,18). Our results are in line with these results, as we are reporting here almost 40% 5-year survival after pneumonectomy in the cohort of patients with locally advanced NSCLC. Moreover, favorable long term outcome was similar after both left and right pneumonectomy.
Even without induction therapy, pneumonectomy is a high risk procedure, with mortality rate reaching 13% (19). After induction therapy, 90-day postpneumonectomy mortality was reported to exceed 20% (10,14). However, in our series, 90-day postoperative mortality was at acceptable level of 10.3%. Discrepancy between these results is probably multifactorial and may result from different surgical technique, experience of the performing surgeons or differences of the evaluated cohorts.
Overall 5-year survival of radically operated patients with mediastinal nodal clearance after induction therapy was reported to be as high as 49% (20). Treatment results depend on several factors, among others tumour histology, pretreatment extent of nodal involvement or radicality and extend of surgical resection. Pneumonectomy after neoadjuvant therapy, like every treatment associated with substantial risk, should be performed only in selected patients. Apart from adequate performance status, lack of significant comorbidities and technical possibility of complete tumour resection, clearance of mediastinal nodal metastases after induction treatment is of great importance for patient selection. Numerous studies have shown that persistent N2 disease after neoadjuvant therapy was associated with a poor prognosis after surgery (3,12,20,21). Still, there are not many studies reporting long-term outcomes, when patient selection for surgical resection was based on the results of mediastinal restaging. In one study, surgical mediastinal restaging after induction treatment and prior to lobectomy was associated with better prognosis than surgical resection without restaging (22). Restaging of the mediastinum after induction treatment may be performed with different techniques, like imaging, endoscopic ultrasound or surgical exploration of the mediastinum, but none is regarded as a standard. Although EBUS-TBNA is an accurate, minimally invasive test for mediastinal restaging, its negative predictive value is low and tumor-negative results need to be validated by surgical staging (6). Remediastinoscopy was reported to be of limited usefulness as a restaging technique (23). We have previously shown that diagnostic yield of TEMLA in restaging of NSCLC was superior to imaging techniques, endoscopic ultrasound and remediastinoscopy (24). To avoid surgical staging in unnecessary cases, our approach was to use consecutively different restaging methods, starting with the least invasive. Patients underwent CT or PET/CT scan to evaluate possible systemic progression of the disease after completion of induction therapy. If imaging studies showed no signs of distant metastases, our initial procedure for restaging of the mediastinum was endoscopic ultrasound (EBUS/EUS). Endoscopic ultrasound was shown to be a good test to confirm the presence of the mediastinal LN metastases and avoid unnecessary surgical mediastinal exploration (6). In case of negative findings of endoscopic ultrasound, patients proceeded to TEMLA and only patients with metastases-free mediastinal LNs after TEMLA underwent pneumonectomy. Patients with persistent N2 disease were referred to definitive oncological treatment. We performed TEMLA in all patients with locally advanced NSCLC after induction treatment. Although less than half of them had confirmed pretreatment mediastinal nodal involvement, the risk of the mediastinal LN metastases in centrally located tumours is high and should be ruled out before pneumonectomy (25). Despite the fact that the restaging was more technically demanding after neoadjuvant treatment, particularly after chemoradiotherapy, no complications occurred in this series. Thus, TEMLA proved to be a safe restaging procedure.
In this study, falsely negative results were found in 5.9% of TEMLAs. Overall 5-year survival of patients who underwent pneumonectomy after falsely negative TEMLA was 25%, as opposed to 39.7% survival of patients operated after true-negative procedure. However, the difference was not statistically significant, possibly due to small size of the cohorts. In other studies, patients operated after positive remedistinoscopy lived significantly shorter than those with true mediastinal clearance (23,26) and their overall 5-year survival was reported to approach 20% (12,27).
In conclusion, the results presented here demonstrate that using invasive mediastinal restaging for precise selection of patients with locally advanced NSCLC with good response to neoadjuvant treatment may lead to good long-term outcomes after pneumonectomy. TEMLA proved to be safe and effective in mediastinal restaging. As perioperative complications were at acceptable level and long-term survival was good, selected patients with locally advanced NSCLC may be treated with pneumonectomy as part of the multimodal therapy when it allows for complete tumour resection that cannot be achieved with lesser procedures.
Limitations
The main limitation of this study is the small sample size. With this cohort size, we were not able to demonstrate the difference in survival of patients with persistent mediastinal LN metastases and patients with mediastinal nodal downstaging. Moreover, as this was a retrospective analysis, the results should optimally be confirmed by appropriately sized prospective study.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sara Ricciardi) for the series “Thoracic Malignancy, Controversies in N Parameter” published in AME Medical Journal. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-21-38/rc
Data Sharing Statement: Available at https://amj.amegroups.com/article/view/10.21037/amj-21-38/dss
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-21-38/coif). The series “Thoracic Malignancy, Controversies in N Parameter” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of our hospital (approval No. 1/2021) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Weder W, Collaud S, Eberhardt WE, et al. Pneumonectomy is a valuable treatment option after neoadjuvant therapy for stage III non-small-cell lung cancer. J Thorac Cardiovasc Surg 2010;139:1424-30. [Crossref] [PubMed]
- Betticher DC, Hsu Schmitz SF, Tötsch M, et al. Mediastinal lymph node clearance after docetaxel-cisplatin neoadjuvant chemotherapy is prognostic of survival in patients with stage IIIA pN2 non-small-cell lung cancer: a multicenter phase II trial. J Clin Oncol 2003;21:1752-9. [Crossref] [PubMed]
- Port JL, Kent MS, Korst RJ, et al. Positron emission tomography scanning poorly predicts response to preoperative chemotherapy in non-small cell lung cancer. Ann Thorac Surg 2004;77:254-9; discussion 259. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Ojha B. Restaging patients with N2 (stage IIIa) non-small cell lung cancer after neoadjuvant chemoradiotherapy: a prospective study. J Thorac Cardiovasc Surg 2006;131:1229-35. [Crossref] [PubMed]
- Herth FJ, Annema JT, Eberhardt R, et al. Endobronchial ultrasound with transbronchial needle aspiration for restaging the mediastinum in lung cancer. J Clin Oncol 2008;26:3346-50. [Crossref] [PubMed]
- Zieliński M. Transcervical extended mediastinal lymphadenectomy: results of staging in two hundred fifty-six patients with non-small cell lung cancer. J Thorac Oncol 2007;2:370-2. [Crossref] [PubMed]
- Asamura H, Naruke T, Tsuchiya R, et al. Bronchopleural fistulas associated with lung cancer operations. Univariate and multivariate analysis of risk factors, management, and outcome. J Thorac Cardiovasc Surg 1992;104:1456-64. [Crossref] [PubMed]
- Amin MB, Edge S, Greene F, et al. editors. AJCC Cancer Staging Manual (8th edition). New York: Springer International Publishing, 2017.
- d'Amato TA, Ashrafi AS, Schuchert MJ, et al. Risk of pneumonectomy after induction therapy for locally advanced non-small cell lung cancer. Ann Thorac Surg 2009;88:1079-85. [Crossref] [PubMed]
- Stamatis G. Risks of neoadjuvant chemotherapy and radiation therapy. Thorac Surg Clin 2008;18:71-80. [Crossref] [PubMed]
- Bueno R, Richards WG, Swanson SJ, et al. Nodal stage after induction therapy for stage IIIA lung cancer determines patient survival. Ann Thorac Surg 2000;70:1826-31. [Crossref] [PubMed]
- Martin J, Ginsberg RJ, Abolhoda A, et al. Morbidity and mortality after neoadjuvant therapy for lung cancer: the risks of right pneumonectomy. Ann Thorac Surg 2001;72:1149-54. [Crossref] [PubMed]
- Doddoli C, Barlesi F, Trousse D, et al. One hundred consecutive pneumonectomies after induction therapy for non-small cell lung cancer: an uncertain balance between risks and benefits. J Thorac Cardiovasc Surg 2005;130:416-25. [Crossref] [PubMed]
- Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [Crossref] [PubMed]
- Gudbjartsson T, Gyllstedt E, Pikwer A, et al. Early surgical results after pneumonectomy for non-small cell lung cancer are not affected by preoperative radiotherapy and chemotherapy. Ann Thorac Surg 2008;86:376-82. [Crossref] [PubMed]
- Yang CJ, Shah SA, Lin BK, et al. Right-Sided Versus Left-Sided Pneumonectomy After Induction Therapy for Non-Small Cell Lung Cancer. Ann Thorac Surg 2019;107:1074-81. [Crossref] [PubMed]
- Daly BD, Fernando HC, Ketchedjian A, et al. Pneumonectomy after high-dose radiation and concurrent chemotherapy for nonsmall cell lung cancer. Ann Thorac Surg 2006;82:227-31. [Crossref] [PubMed]
- Licker M, Spiliopoulos A, Frey JG, et al. Risk factors for early mortality and major complications following pneumonectomy for non-small cell carcinoma of the lung. Chest 2002;121:1890-7. [Crossref] [PubMed]
- Decaluwé H, De Leyn P, Vansteenkiste J, et al. Surgical multimodality treatment for baseline resectable stage IIIA-N2 non-small cell lung cancer. Degree of mediastinal lymph node involvement and impact on survival. Eur J Cardiothorac Surg 2009;36:433-9. [Crossref] [PubMed]
- Voltolini L, Luzzi L, Ghiribelli C, et al. Results of induction chemotherapy followed by surgical resection in patients with stage IIIA (N2) non-small cell lung cancer: the importance of the nodal down-staging after chemotherapy. Eur J Cardiothorac Surg 2001;20:1106-12. [Crossref] [PubMed]
- Yang CF, Adil SM, Anderson KL, et al. Impact of patient selection and treatment strategies on outcomes after lobectomy for biopsy-proven stage IIIA pN2 non-small cell lung cancer. Eur J Cardiothorac Surg 2016;49:1607-13. [Crossref] [PubMed]
- Stamatis G, Fechner S, Hillejan L, et al. Repeat mediastinoscopy as a restaging procedure. Pneumologie 2005;59:862-6. [Crossref] [PubMed]
- Zieliński M, Hauer L, Hauer J, et al. Non-small-cell lung cancer restaging with transcervical extended mediastinal lymphadenectomy. Eur J Cardiothorac Surg 2010;37:776-80. [Crossref] [PubMed]
- Moulla Y, Gradistanac T, Wittekind C, et al. Predictive risk factors for lymph node metastasis in patients with resected non-small cell lung cancer: a case control study. J Cardiothorac Surg 2019;14:11. [Crossref] [PubMed]
- Call S, Rami-Porta R, Obiols C, et al. Repeat mediastinoscopy in all its indications: experience with 96 patients and 101 procedures. Eur J Cardiothorac Surg 2011;39:1022-7. [Crossref] [PubMed]
- Martin J, Ginsberg RJ, Venkatraman ES, et al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. J Clin Oncol 2002;20:1989-95. [Crossref] [PubMed]
Cite this article as: Gwóźdź P, Zieliński M. Mediastinal restaging with transcervical extended mediastinal lymphadenectomy in patients with locally advanced non-small cell lung cancer treated with pneumonectomy. AME Med J 2022;7:22.




