
Cholestatic liver injury from Ganoderma lucidum coffee extract—a case report
Introduction
Drug-induced liver injury (DILI) poses an exceptionally challenging diagnostic situation for physicians because of an expansive array of prescription drugs (1). Recent data reports an annual incidence of DILI between 15–20 per 100,000 in the general population, although the numbers could be more significant owing to gross under-reporting from a lack of awareness and improper record keeping (2-4). Over the last decade, we have also witnessed the rise of herbal consumption, resulting in an upsurge trend of herb-induced liver injury (HILI) incidence from 7% in 2004–2005 to 20% in 2013–2014 (5,6). This data is projected to rise even further as traditional remedies become more widely available in various formulations or as additives in food and beverages. Of note, the public is generally more receptive to complementary and alternative medicines, believing that the treatment used is natural and, thus, potentially safe compared to licensed medications (7). Although some herbal products may be safe when consumed sparingly, others may not be as forgiving (8,9).
We recently encountered a patient presenting with cholestatic liver injury following prolonged Ganoderma lucidum ingestion, purportedly as an energy booster in his desire to lose weight. The substance was premixed into coffee powder as an active ingredient and sold in a box of 20 sachets. To the best of our knowledge, this is the first recorded hepatotoxicity event caused by Ganoderma lucidum extract in a beverage. We present the following case in accordance with the CARE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-22-27/rc).
Case presentation
Our patient is a 19-year-old man who presented with complaints of painless jaundice, tea-coloured urine, pale stools, worsening pruritus, appetite loss, and vomiting for 1 week. There was also a 6-month history of intentional weight loss of 20 kg before the current presentation. He reported no fever, diarrhoea, abdominal pain or swelling, neuropsychiatric and B symptoms. Aside from this, he has no past medical and surgical issues and has not travelled outside the country lately. He had been well and denied consuming over-the-counter pills, traditional medications, recreational and illicit drugs, protein shakes or anabolic steroids, supplements, antibiotics or painkillers. He does not use drugs or alcohol and has no history of sexual risk behaviours.
Clinical examination demonstrates deep jaundice and multiple scratch marks with no cutaneous stigmata of chronic liver disease and no signs of encephalopathy. The blood pressure was 137/60 mmHg, pulse rate 90 beats/min, respiratory rate 16 breaths per minute, temperature 36.5 ℃, and oxygen saturation 98% on room air. Other systemic examinations were unremarkable.
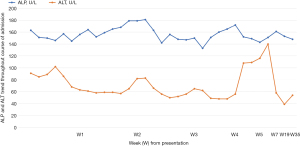
Notable investigations revealed elevated total bilirubin, which peaks at its highest at week 2 of symptom onset, measuring 543.0 mmol/L (normal range, <21 mmol/L) with predominant direct hyperbilirubinemia, 433.9 mmol/L (normal range, 0–5 mmol/L) (Figure 1). The alanine transaminase (ALT) ranges from 56–102 U/L (normal range, 0–41 U/L), whereas the aspartate transaminase (AST) ranges between 48–110 U/L (normal range, 0–34 U/L). On the other hand, the alkaline phosphatase (ALP) did not fluctuate much, with the highest recorded value at 181 U/L (normal range, 40–130 U/L) (Figure 2). The albumin, however, dips to its lowest at 23 g/L (normal range, 34–48 g/L) though the globulin remains relatively stable. Routine first-line blood investigations, including complete blood count, gamma-glutamyl transferase levels, renal profile, coagulation profile, electrolytes, infective and inflammatory panels, were otherwise normal. An exhaustive panel of second-line investigations sent to work up for haematological malignancy, tuberculosis, viral hepatitis (including hepatitis A and E), autoimmune hepatitis, Wilson’s disease, haemochromatosis and alpha-1 antitrypsin deficiency were negative. Concerning his hepatitis B status, there was serological evidence of a previous infection which has now resolved (positive hepatitis B core antibody, positive hepatitis B surface antibody, negative hepatitis B surface antigen and undetectable viral load).
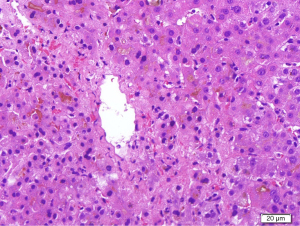
Multiple radiological investigations performed, including transthoracic echocardiography, hepatobiliary ultrasound, computed tomography of the abdomen, and diagnostic endoscopic ultrasound, were normal. An upper gastrointestinal endoscopy and colonoscopy were likewise unremarkable. Following this, a liver biopsy performed at week 4 from initial presenting complaints demonstrated marked perivenular (Zone 3) hepatocanalicular cholestasis, which was supportive but not conclusive for DILI (Figure 3). There was also Stage 4 fibrosis (modified hepatitis activity index) marked by portal-to-portal bridging fibrosis from previous viral hepatitis B insult, although there was no biochemical, serological and virological evidence to support a recent acute hepatitis B flare.
Somehow, with adequate hydration and dietitian-prescribed nutrition throughout his admission, his direct bilirubin levels improved to 126.8 mmol/L (normal range, 0–5 mmol/L). Moreover, his oral intake got better with gradual weight regaining. In addressing his pruritus, we commenced him on antihistamines and subsequently switched to oral ursodeoxycholic acid and cholestyramine when the primary agents did not help to relieve the itchiness.
Our patient returned 2 weeks later to our outpatient clinic and reported significant improvements in his symptoms. When explaining that the liver biopsy demonstrated findings probable for HILI, he became defensive and vehemently denied taking any supplements. As we delved into his recent voluntary weight loss decision, we began to question him on his routines and finally discovered that he has been taking a premixed coffee with Ganoderma lucidum (Lingzhi) extract. From the patient’s perspective, this was not a supplement as it was just like any other coffee product to provide an additional energy boost during his intermittent fasting routine in addition to helping him lose a considerable amount of weight. For a complete timeline of events, we illustrated this in a graphical format for ease of reference (Figure 4).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The use of traditional medicine in various medical conditions and consumption of herbal products for improvements to general well-being or as weight loss aids are common in Asia. Compared to conventional medicine, these traditional treatments stem from ancient roots traced thousands of years ago from narrative or visual records handed down from generation to generation. Such time-honoured traditional practice that has existed since its early days enjoys full approval and wide acceptance from various religious, societal and cultural backgrounds (10). Unlike modern medicine, it escapes from rigorous scientific research and stringent quality control and circulates widely among the public through social media like the proverbial word-of-mouth. Indeed, there is a dearth of publications from East Asian countries on the true incidence of traditional medicine consumption owing to under-reporting, unregulated prescription habits, and the scarcity of post-marketing surveillance. On the flip side, there is a better account of the incidence and prevalence of hepatotoxicity from HILI, which ranges broadly from 2% to 30% in countries like China, Korea, Japan, Singapore and India (10). Closer to home in Malaysia, recent reports discovered an astounding 11.24% incidence of hepatic adverse drug reactions resulting from herb consumption, taking the second spot after anti-tuberculous drugs (11).
There are two means by which hepatotoxicity may occur in DILI and HILI: intrinsic (drug-related) and idiosyncratic (interaction between the drug and patient factors). The intrinsic mechanism is more predictable owing to its direct dose-dependent effect, occurring within hours to days. Conversely, idiosyncratic reactions are dose-independent and highly unanticipated, with variable onset ranging from several days to weeks and even months (12). Despite sharing identical mechanisms in causing liver injury and clinical manifestations, how HILI behaves is more varied owing to the inconsistent dosage, formulation and possible contamination during preparation, manufacturing, production and storage. Given that most herbs sold and consumed do not lead to overt adverse events, we believe our patient might have developed an idiosyncratic reaction towards Ganoderma lucidum. Nevertheless, it is also possible that an intrinsic mechanism might have taken place owing to the prolonged consumption resulting in a cumulative higher dose altogether. Regardless of the putative hepatotoxicity mechanism, this does not alter the subsequent management.
In this case, our patient’s R-factor was in keeping with cholestatic liver injury, thus, prompting further evaluation along the diagnostic imaging pathway (13). Per established recommendations and guidelines, we then applied the updated Roussel Uclaf Causality Assessment Method (RUCAM) scale for a more systematic and thorough appraisal to ensure all competing etiologies were ruled out (14). Of note, the utility of RUCAM has helped establish causality assessment in 95,885 cases from 1993 until 2020, with 81,856 and 14,029 cases attributed to DILI and HILI, respectively, rendering it an indispensable tool for many gastroenterology and hepatology societies worldwide in advising its use (15,16). In our scenario, the patient’s RUCAM score was 8, thus translating to probable causality, which drives the desire for a histopathological diagnosis.
The liver biopsy revealed marked hepatocanalicular cholestasis, which we anticipated while demonstrating significant portal-to-portal bridging fibrosis, a histological hallmark reminiscent of underlying chronic liver disease. Taken together with serological evidence of a functional cure for Hepatitis B infection, we postulated the background liver fibrosis to be directly related to previous chronic hepatitis B infection. The current herb-induced cholestatic liver injury would have unlikely contributed to such an extent of liver fibrosis owing to different pathogenesis and its known reversibility (17). Here lies the probable explanation we were looking for regarding the likeliest liver injury mechanism; intrinsic versus idiosyncratic. As the product he consumed is also widely available and taken without reported hepatotoxicity encounters, we believe that a considerable background of liver fibrosis would have made him more susceptible to developing an idiosyncratic reaction. With relevance to our discussion on weight loss supplements, we would also like to highlight the glaring similarities of liver injury pattern from androgenic anabolic steroids, which is known to manifest with marked hyperbilirubinemia but otherwise, normal or minimally elevated ALP with histology demonstrating reversible canalicular and hepatocellular cholestasis. After all, anabolic steroid ingestion is not uncommon in healthy individuals aiming to lose weight. We considered the potential consumption of such compound; however, our patient’s weight loss journey did not extend towards exercising and fitness, in which case such supplement intake would have been more fitting (17).
The potential pitfall that we encountered was that our patient felt that the beverage premixed with Ganoderma lucidum was not, in his understanding, a traditional medicine, thus, resulting in the omission of a vital clue to help us in our diagnostic workup. Furthermore, the advertised reputation of Lingzhi consumption, namely, its cytoprotective, hepatoprotective, anti-neoplastic and anti-oxidant properties, are far more prevalent than published adverse events (18). The common problem amongst the local community worldwide is the dichotomy between preferring anecdotal proof over scientific evidence as complementary and alternative medicines are often more attractive due to their presumed ‘non-toxic natural’ origins. Such perception can backfire if people are unaware that they may have undiagnosed chronic liver disease. To drive home the point on the lack of published adverse events, hepatotoxicity from Ganoderma lucidum appeared in only two case reports, with the latest occurring in 2005. Both cases presented with a mixed hepatocellular and cholestatic liver injury following a month’s ingestion of Lingzhi in its powder form, and while one survived, the second patient succumbed to fulminant hepatic failure. In these two cases, the patients consumed traditionally boiled Lingzhi slices as health supplements with no untoward problems until they switched to the capsule containing 400 mg of Lingzhi extract. Like our patient, an extensive line of investigations performed was unremarkable, though, unlike in our case, the perpetrating agent was made evident from the beginning (19,20).
We believe there could be an underreporting of events, with cases remaining undiagnosed from the lack of awareness. Nevertheless, the European Association for the Study of the Liver (EASL) DILI guidelines have listed Ganoderma lucidum, amongst other Asian medicinal compounds, as a potential hepatotoxic agent with an acute hepatocellular injury pattern. Our case is the first reported HILI incident due to ingesting Ganoderma lucidum extract in a premixed beverage, the latter of which is an increasingly rampant occurrence in our community owing to the rising desire for alternative ‘natural’ health supplements, its wide availability without prescription and significantly cheaper cost. Though we could only establish a probable cause here, a detailed drug profiling did not reveal any other concomitant medications consumed by our patient. Furthermore, upon cessation of the drug a few days before admission, his liver enzymes gradually normalized without any pharmacological intervention.
Despite a growing list of established hepatotoxins on EASL, a significant knowledge gap still exists in our understanding of HILI, which ranges across the demand for robust epidemiological data, a more in-depth understanding of the pathogenesis between drug-host interactions, and the potential development of biomarkers to assist in diagnosis and prognosis, as well as to look into specific treatment to address the various clinical outcomes. The way forward is to develop a foolproof reporting system for potential hepatotoxins to be furnished to regulatory bodies, replete with relevant information to permit further investigations and subsequent follow-ups.
Until then, our strategy involves elaborate history taking, considering all aspects of potential hepatotoxins that can be summarized in a checklist to avoid oversight. If suspicions arise during patient care, one should not hesitate to revisit the history or aim to gather more input from primary caregivers or relatives who are close to the patient. Complementing this with the R-factor and RUCAM scale is indispensable as it guides subsequent and appropriate investigative pathways.
Acknowledgments
We express our sincere gratitude and appreciation by acknowledging our team of ward doctors and nurses who took care of our patient while he was admitted to the gastroenterology ward in both the Duchess of Kent Hospital in Sandakan and Queen Elizabeth Hospital in Kota Kinabalu, Sabah.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-22-27/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-27/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Real M, Barnhill MS, Higley C, et al. Drug-Induced Liver Injury: Highlights of the Recent Literature. Drug Saf 2019;42:365-87. [Crossref] [PubMed]
- Chalasani N, Bonkovsky HL, Fontana R, et al. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology 2015;148:1340-52.e7. [Crossref] [PubMed]
- Tujios SR, Lee WM. Acute liver failure induced by idiosyncratic reaction to drugs: Challenges in diagnosis and therapy. Liver Int 2018;38:6-14. [Crossref] [PubMed]
- Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol 2012;18:249-57. [Crossref] [PubMed]
- Navarro VJ, Khan I, Björnsson E, et al. Liver injury from herbal and dietary supplements. Hepatology 2017;65:363-73. [Crossref] [PubMed]
- Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013;144:1419-25, 1425.e1-3; quiz e19-20.
- Amadi CN, Orisakwe OE. Herb-Induced Liver Injuries in Developing Nations: An Update. Toxics 2018;6:24. [Crossref] [PubMed]
- Schoepfer AM, Engel A, Fattinger K, et al. Herbal does not mean innocuous: ten cases of severe hepatotoxicity associated with dietary supplements from Herbalife products. J Hepatol 2007;47:521-6. [Crossref] [PubMed]
- Ballotin VR, Bigarella LG, Brandão ABM, et al. Herb-induced liver injury: Systematic review and meta-analysis. World J Clin Cases 2021;9:5490-513. [Crossref] [PubMed]
- Philips CA, Augustine P, Rajesh S, et al. Complementary and Alternative Medicine-related Drug-induced Liver Injury in Asia. J Clin Transl Hepatol 2019;7:263-74. [Crossref] [PubMed]
- Lee FY, Wong HS, Chan HK, et al. Hepatic adverse drug reactions in Malaysia: An 18-year review of the national centralized reporting system. Pharmacoepidemiol Drug Saf 2020;29:1669-79. [Crossref] [PubMed]
- Roth RA, Ganey PE. Intrinsic versus idiosyncratic drug-induced hepatotoxicity--two villains or one? J Pharmacol Exp Ther 2010;332:692-7. [Crossref] [PubMed]
- Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014;109:950-66; quiz 967. [Crossref] [PubMed]
- Danan G, Teschke R. RUCAM in Drug and Herb Induced Liver Injury: The Update. Int J Mol Sci 2015;17:14. [Crossref] [PubMed]
- Teschke R, Danan G. Worldwide Use of RUCAM for Causality Assessment in 81,856 Idiosyncratic DILI and 14,029 HILI Cases Published 1993-Mid 2020: A Comprehensive Analysis. Medicines (Basel) 2020;7:62. [Crossref] [PubMed]
- Teschke R. Idiosyncratic DILI: Analysis of 46,266 Cases Assessed for Causality by RUCAM and Published From 2014 to Early 2019. Front Pharmacol 2019;10:730. [Crossref] [PubMed]
- Stolz A, Navarro V, Hayashi PH, et al. Severe and protracted cholestasis in 44 young men taking bodybuilding supplements: assessment of genetic, clinical and chemical risk factors. Aliment Pharmacol Ther 2019;49:1195-204. [Crossref] [PubMed]
- Shiao MS. Natural products of the medicinal fungus Ganoderma lucidum: occurrence, biological activities, and pharmacological functions. Chem Rec 2003;3:172-80. [Crossref] [PubMed]
- Yuen MF, Ip P, Ng WK, et al. Hepatotoxicity due to a formulation of Ganoderma lucidum (lingzhi). J Hepatol 2004;41:686-7. [Crossref] [PubMed]
- Wanmuang H, Leopairut J, Kositchaiwat C, et al. Fatal fulminant hepatitis associated with Ganoderma lucidum (Lingzhi) mushroom powder. J Med Assoc Thai 2007;90:179-81. [PubMed]
Cite this article as: Tay YZ, Pan AF, Chiam KH. Cholestatic liver injury from Ganoderma lucidum coffee extract—a case report. AME Med J 2022;7:29.



