
Wilson disease: more complex than just simply a copper overload condition?—a narrative review
Introduction
The present review collects data from literature and combines them with an own perspective of the disease obtained by long standing personal experience with Wilson disease patients.
It should not be considered as a complete textbook-like collection about all the facts on Wilson disease which would burst the allowed space for the contribution. For this we refer to a respective representative publication (1). The subject is not even a systematic review of which so many already exist or a meta-analysis. Intention of the article is the collection of data to support the view that Wilson disease is a complex multifaceted and unique copper overload condition, where intracellular accumulation of toxic free copper, but not total copper, causes organ damage. Therapy should therefore be directed to reduce copper toxicity. The paper is to stimulate scientists to drill further into the pathophysiology of this obscure disease with the intention to help affected patients. We present the following article in accordance with the Narrative Review reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-22-24/rc).
Methods
Search process for relevant papers about studies reporting critical mechanisms inducing cell injury and strategies for prevention
For this narrative review, we searched the PubMed database for studies published between years 1980–2022 using the search terms “mechanism of liver injury in Wilson disease”, “therapeutic strategies”, and “monitoring and outcome”. Papers identified by these search terms with no further inclusion or exclusion criteria were screened for information providing new aspects about the biochemical disease process and the role of copper in the pathogenesis of Wilson disease.
Key Content and Findings
Physiology of copper metabolism
Copper (Cu) is a trace element essential in electron transfer reaction which is required for cellular respiration including handling of oxygen and free radicals (2). In food it is present mostly in its cupric form (Cu2+) and the recommended daily allowance is age-dependent, e.g., in adults 900 µg/day (3). About 50% is absorbed for which it is reduced to Cu+ (cuprous form) outside of mucosal cells (4). The mechanistic of copper physiology is depicted in the cartoon of Figure 1. Only free Cu+ can be translated across membranes. However, it is cell toxic due to free radical formation and therefore must be bound to proteins for cellular protection (5). Uptake into cells is mediated by a specific, high affinity transport system, the Cu transporter 1 (CTR1) (6-9). Within cells it is handled to the antioxidant Cu chaperone (ATOX1), which directs Cu+ to Cu-transporting P-type ATPases (10,11). In intestine the predominant Menkes disease protein, the ATPase copper transporter alpha (ATP7A) translocates Cu+ out of mucosal cells (Figure 1). The external loop of ATP7A is rich in histidine and methionine residues which are essential for dephosphorylation to release copper (12). At the basolateral side of mucosal cells transported Cu+ is reduced to Cu2+ and taken up by the amino acid histidine. It is reported that Cu2+ in blood is shuttled between histidine for cellular uptake and release and on the other hand albumin for transportation, both constituting the non-ceruloplasmin bound “free” Cu fraction (13-15). It means that Cu2+ bound to albumin for transport in blood can be released at cellular uptake sites by accumulated histidines or histidine residues at the transporter CTR1. Free copper also represents the Cu, which is secreted in urine because histidine bound Cu2+ can be filtered by the glomerula and accounts for increased urinary Cu excretion in Wilson disease.

The liver demands most of the absorbed Cu for incorporation in enzymes controlling metabolism, for ceruloplasmin synthesis and for biliary excretion which is the control mechanism to maintain a balanced Cu level (16-18). Uptake in hepatocytes occurs via CTR1. Its external loop allows binding of Cu2+ by histidine containing domains followed by oxidation to Cu+ for transportation into the cell (15). Intracellularly Cu+ is handled to ATOX1 for delivery to the Wilson disease protein ATP7B located on the trans-Golgi network for transmembrane transfer and release to the Golgi lumen. Similar as with ATP7A in mucosal cells, release is mediated by dephosphorylation utilizing histidine and methionine rich domains (19,20).
Translocated Cu2+ is soluble in the acid milieu of the Golgi lumen and therefore accessible for Cu2+ requiring enzymes, in particular ceruloplasmin (Figure 1). Step by step apoceruloplasmin is loaded with 6 atoms of Cu2+ (21,22). Holoceruloplasmin is functional and packed into vesicles, the content of which is released at the basolateral side to the circulation, representing the predominant Cu-binding protein in blood (95% of Cu found in plasma) (21-23). It is required in the organism for oxidation and cellular iron excretion (21,23). Non-Cu-loaded apoceruloplasmin is also secreted in blood. Its function needs further exploration (22). ATOX1 bound Cu+ can also be transferred to other cytosolic enzymes requiring Cu+ as well as metallothionein, a cysteine rich protein which can store to a certain extent excessive Cu+ (Figure 1) (24-26).
In situation of an increase in hepatocellular cytosolic Cu, ATP7B localizes to an acidified vesicular compartment near the canalicular membrane to accumulate Cu2+ for subsequent excretion into bile (Figure 1) (27-30). The mechanism of biliary release is not completely elucidated. The presence of the Cu metabolism gene MURR1 also known as COMM domain-containing protein 1 (COMMD1) plays a catalytic roll in this process (30,31). Once Cu is in bile it forms an unabsorbable complex, possibly with certain bile acids, which is eliminated in stool (Figure 1) (30).
Pathophysiology of Wilson disease and its impact of disease manifestation
Cu overload is most impressively seen in Wilson disease where mutations only of the ATP7B gene are causative (about 1,400 reported mutations) (31-33). Mutations result in malfunction of ATP7B (Figure 1). Due to impaired influx in the trans-Golgi network Cu is not loaded to apoceruloplasmin. Consequently, holoceruloplasmin is not or only marginally produced disturbing its metabolic responsibilities. In contrast to low ceruloplasmin levels observed in Wilson disease, the very rare genetic disorder of aceruloplasminemia presents with a more severe neurodegenerative phenotype including ataxia, unvoluntary movements, dystonia, tremor and chorea (34). Underlying defect is the disturbance of cellular iron release with intracellular iron accumulation, but iron deficiency in blood (21,23).
The other pathophysiologically more relevant consequence of ATP7B malfunction is impairment of Cu excretion in bile resulting in hepatocellular accumulation, although bile flow is maintained. Low biliary Cu excretion results in view of the negligible urinary excretion of free Cu in a positive Cu balance. In early stages the liver deposits the Cu burden within metallothionein, the synthesis of which is stimulated by Cu (Figure 1) (35). At this stage liver injury is not expected although hepatic Cu content may be highest over the course of the disease. When the metallothionein storage capacity is exhausted, the hepatocyte has the option to stop further influx and to export Cu+ through CTR1 back to the systemic circulation. This Cu2+ bound to histidine or albumin, the free Cu pool, remains in the circulation for delivery to other organs.
Later in the course of the disease excessive hepatic copper is distributed to intracellular Cu storing organelles, which could be lysosomes (Figure 2) (36). With time the lysosomal storage capacity is also exhausted, and the organelle membrane may disrupt by free radical attack (Figure 2) (37). Liver injury with elevated transaminases occurs. The process is a slowly progressing disorder involving repair mechanisms eventually leading to fibrosis and cirrhosis. If the content of bursted lysosomes containing Cu+ and acid is rapidly released to cytoplasm, fulminant hepatic failure may evolve, triggered by an apoptotic shut down (38,39). The consequent cell destruction leads to massive release of Cu+ to the circulation which may not even be bound anymore to the histidine/albumin complexes, at least not with high affinity. Excessive free Cu+ release also causes attack of red cell membranes and, thus, hemolysis (40). Cu in urine increases dramatically. However, increased urinary Cu excretion may not be seen in fulminant hepatic failure due to a concomitant hepatorenal syndrome with kidney failure and anuria.
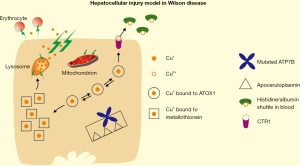
The free Cu is distributed via the histidine-albumin shuttle throughout the organism. In brain uptake of excess Cu leads to neuronal degeneration, mostly in basal ganglia. It affects the fine motoric movements and coordination, impairing speech, swallowing and hand writing (41). Moreover, psychiatric disturbances are recognized (42). It is discussed whether this is only due to copper overload or in part also to iron accumulation, in particular in cases with severe neurologic manifestation, because ceruloplasmin—the iron exporter—is decreased (23,43). Indeed, brain iron was found to be increased, however, it was argued not to be primary, but secondary to tissue damage (44).
From kidneys the free Cu is excreted in urine. Under physiologic conditions where 95% of serum Cu is tightly bound to ceruloplasmin, only trace amounts appear in urine. However, in untreated Wilson disease cases more than 60 µg/day are released. The amount can be much higher depending on the free Cu load in blood.
Other rare manifestations of Wilson disease concern the heart revealing cardiomyopathy, joints revealing arthritis and kidneys revealing renal tubular acidosis with predisposition for stone formation (45). The accumulation of Cu in the cornea leads to the pathognomonic Kayser-Fleischer rings (17).
The presentation of Wilson disease reveals a predominant hepatic, predominant neurologic, a mixed or asymptomatic phenotype. The correlation to a certain ATP7B genotype failed at first sight (46). However, recent reports challenged this view and it not excluded that some associations may be found in near future (47-49). Therefore, investigators also sought for disease amplifying conditions, but, unfortunately, without any success until today. Candidates of the involved known proteins of Cu metabolism could not be identified to modify symptomatology and disease progression (46). In particular, asymptomatic patients or those who developed symptoms rather late in life are of interest. If failure of biliary excretion is essential for pathogenesis, the question arises whether there are ATP7B independent routes of biliary copper excretion. Alternatively, it is likely that Wilson disease mutations cause only a less functional or lower quantity of ATP7B, which slow down metabolism without extinction of the responsible pathways completely. It may be similar as observed in newborns where the ATP7B-dependent Cu excretion pathway is not operative due to lack of bile flow, but no hepatocellular injury occurs (50,51). The switch of just Cu overload, where Cu+ is bound to metallothionein, to Cu-induced hepatocellular injury is not well defined. However, the answer to this question will be of help for therapy of Wilson disease. In this context it is of interest that a gender effect is recognized in Wilson disease (52,53). There is a slight male predominance in disease manifestation with little difference in neuropsychiatric symptoms, but females tend to have more hepatic manifestation (53).
It is not excluded that other liver tackled disorders have impact on presentation of Wilson disease, e.g., primary hemochromatosis, α1-antitrypsin deficiency or alcoholic and non-alcoholic steatohepatitis (54). As an example, we recently identified two siblings with Wilson disease who had an autoimmune disorder disposition with elevation of anti-nuclear antibodies (ANA) and anti-smooth muscle antibodies, although the IgG level was in both patients in the normal range (55). Antibodies were already elevated at time of diagnosis. Both showed a hepatic type of Wilson disease. Treatment with D-penicillamine resulted in decoppering with normal urinary Cu excretion after one year. Over the next following 10 years, transaminases fluctuated between 50 and 500 U/L. At peak of transaminases a short-term (4 weeks) application of 20 mg prednisolone reduced these by 80% which indicates that hyperimmunity but not Cu overload induced the inflammatory phenotype. Although such case reports are not conclusive evidence for a new disease entity, it may open the treating physician for the option that underlying conditions may modulate disease presentation.
Cholestasis and idiopathic childhood cirrhosis, two other copper overload conditions
When biliary Cu excretion is impaired as under cholestatic conditions, Cu accumulates in hepatocytes (30,56-58). However, the degree of Cu overload is less severe and ceruloplasmin metabolism remains unaltered (59). Moreover, the copper chelator D-penicillamine, which is helpful in Wilson disease, does not have therapeutic impact in reversing cholestasis induced liver injury (60). Intra- and extrahepatic cholestasis can be complicated by inflammation, the cholangitis. In this case laboratory evaluation shows beside cholestasis (elevation of bilirubin, alkaline phosphatase, γ-glutamyltransferase) also elevation of transaminases (i.e., alanine aminotransferase, aspartate aminotransferase).
Cholangitis is attributed to accumulation of too many and toxic bile acids in hepatocytes. Whether the inflammatory phenotype of hepatocytes could also be due to accumulation of non-biliary secreted Cu, reflects an interesting hypothesis. However, intrahepatocellular Cu levels are not excessive and neutralizing metallothionein is operative.
As mentioned before, dietary Cu overload may accelerate liver injury in Wilson disease. As another—even more dramatic example—the idiopathic childhood cirrhosis is a rapid and progressive disorder with a marked increase in hepatic Cu. It was initially described in India in children already by 2 years of age (61,62). Serum ceruloplasmin is normal or even elevated. Epidemiologic studies revealed an increase of Cu content in the diet of affected children. However, in some families an autosomal recessive inheritance with incomplete penetrance was found and genetic abnormalities, also in ATP7B gene, were registered (31,63). The pathogenesis of the disease remains obscure. Therapy with D-penicillamine is effective in many cases and liver transplantation can be curative.
Diagnosis
Diagnosis of Wilson disease is often a challenge. Therefore, a diagnostic algorithm has been developed (Leipzig Score) which is highly reliable and applicable in daily practice (39). In Table 1 the threshold laboratory parameters and the probability of Wilson disease according to the clinical and laboratory findings is summarized. According to pathophysiology, key laboratory parameters for Wilson disease are low ceruloplasmin and, thus, total Cu in blood (Table 1) (39).
Table 1
| Criteria/clinical symptoms | Data/score |
|---|---|
| A: Threshold laboratory values for Wilson disease | |
| Serum copper | <70 µg/dL (12 µmol/L) |
| Ceruloplasmin | <0.2 g/L |
| Urinary copper | >60 µg/day (>0.94 µmol/day) |
| Free copper (calculated) | >15 µg/dL (>0.23 µmol/dL) |
| D-penicillamine 500-induced urinary copper | >1,600 µg/day |
| Liver copper | >250 µg/g dry weight (>4 µmol/g) |
| B: Wilson disease scoring system (Leipzig score) | |
| Kayser-Fleischer rings | Absent: 0 Present: 2 |
| Neurological symptoms | Absent: 0 Present: 1 Severe: 2 |
| Ceruloplasmin | Normal (>0.2 g/L): 0 0.1–0.2 (g/L): 1 <0.1 (g/L): 2 |
| Hemolysis | Absent: 0 Present: 1 |
| Liver copper | <0.8 µmol/g: −1 0.8–4 µmol/g: 1 >4 µmol/g: 2 Rhodanine-positive granules (if no quantitative liver copper available): 1 |
| Urinary copper | Normal: 0 60–120 µg/day: 1 >120 µg/day: 2 Normal, but >300 µg/day after 2×0.5 g D-penicillamine: 2 |
| Mutation analysis | No mutation: 0 On one chromosome: 1 On both chromosomes: 4 |
| Evaluation of total score | 2 or less: diagnosis very unlikely 3: diagnosis possible 4 or more: diagnosis established |
*, all data were taken from (39).
The direct measurement of pathognomonic elevated free Cu (60–80% of serum Cu in Wilson disease!) is nowadays possible as non-ceruloplasmin bound copper (NCC) or relative exchangeable copper (64,65). It is of high diagnostic value, also for control of therapy. However, test performance is still a technical challenge for some routine laboratories and, thus, unfortunately not widely available (66).
However, at low ceruloplasmin concentration (<0.1 g/L) there is a simple formula by which free Cu is approximately estimated (39,67):
The value is useful, but not 100% reliable (67). At ceruloplasmin >0.2 g/L it fails. Due to the high rate of false positive results is not generally recommended (39).
When kidney function is normal, the amount of Cu in urine (24 h collection) is very sensitive and specific to diagnose Wilson disease (>60 µg Cu/day). It represents the filterable free Cu fraction. Thus, urinary Cu reflects the pathogenetic player of organ manifestation in Wilson disease. It is also very useful to control efficacy of the executed therapy removing free Cu from blood. Accordingly, aim of therapy is to suppress urinary Cu excretion below 60 µg/day. For measurement chelator therapy must be paused for about 48 h because they induce artificially excessive copper excretion. Therefore, in case chelator therapy is continued, values of 200–500 µg/day are desirable (68).
Clinically the Kayser-Fleischer ring is a robust criterion for Wilson disease. It is present in almost 100% of neurologic Wilson disease. This is also true for the combination of neurologic symptoms showing motoric disturbances (mostly tremor, speaking and swallowing difficulties, micrographia with small or cramped handwriting) and signs of liver disease (clinical/ultrasonographic features and laboratory elevation of transaminases and parameters of liver dysfunction). It is less frequent in hepatic and pre-/asymptomatic patients (39).
In unclear cases it is still recommended to perform a liver biopsy and to determine the Cu content in dry weight, accounting in normal liver 15–55 µg/g. A measurement of >250 µg/g is pathognomonic for Wilson disease. It has been reported that already a Cu content of 75 µg/g is suggestive for Wilson disease with a specificity of 95.4% and a sensitivity of 96.5% (69).
Another diagnostic criterion is the identification of disease-causing mutations in the ATP7B gene. Due to the autosomal recessive trait compound heterozygous patients are predominant. The number of newly discovered mutations constantly increases up to about 1,400 at present, located in coding, intronic and regulatory sequences, which all affect the function of ATP7B or its abundance in hepatocytes (31-33). The global incidence is 1:30,000 with symptomatic presentation mostly between ages of 5 and 35 (70-73). In case just one mutation is detectable, a heterozygous carrier is suspected with an incidence of 1:5,000 (70-73). They often have slightly depressed serum Cu and ceruloplasmin levels and a mild increase in urinary Cu. However, cohort studies showed no statistical differences between healthy controls and heterozygous carriers, meaning that they do not develop Wilson disease (74).
Currently also the ATP7B peptide assessment is proposed for diagnosis (75). Moreover, the radiocopper test is very reliable to detect homozygous and heterozygous mutation carriers for the Wilson disease gene (76). It is frequently applied in Poland, but due to logistic shortcomings not widely available elsewhere. In addition, the evaluation of Wilson disease with 18F-FDG PET/CT is of high relevance to detect cerebral copper deposition (77).
Therapy
Biliary Cu excretion is the only regulative mechanism to maintain a balanced Cu level in the organism (Figure 1). Therefore, dietary Cu overload should be avoided in Wilson disease, because biliary Cu excretion is impaired. Cu rich foods are cacao, chocolate, nuts, beans, mushrooms, shellfish, raisins and innards like liver, brain and kidney (78). Even drinking excessive tap water from Cu tube installation can increase the Cu burden.
The ultimate goal of the therapy is to reverse Cu induced cellular injury, mainly improvement of hepatic and neurologic manifestation. One may think that it is the removal of Cu from the organism. However, this may only partially be achievable due to the underlying pathophysiology of the disease.
At present there are three, almost equally effective therapies available: zinc, D-penicillamine and trientine. The latter should only be chosen when zinc and D-penicillamine therapy failed. D-penicillamine and trientine are chelators, which operate in blood to bind free Cu for excretion in urine. Thus, they reduce the harmful free Cu to avoid its distribution to organs, including liver and brain. However, a calculation of their capacity to remove Cu from the organism failed to show a significant effect (67). On the other hand, it was shown that both chelators induce metallothionein synthesis, which is known to act as a Cu+ binding protein neutralizing its cellular toxicity (79-82). In intestine, where the chelators must pass the mucosal cells, it is likely that this intracellular induction can occur. Then mucosal cell Cu+ bound to metallothionein is lost in stool with shedding of these cells after 1.4 days. Since both chelators cannot easily enter parenchymal cells in liver and brain, it is questionable whether induction of metallothionein occurs there.
In comparison, zinc acts as strong metallothionein inducer in mucosal cells inhibiting absorption but is also taken up by hepatocytes for further metallothionein synthesis stimulation (67,83). Induction of urinary release of free Cu seems not to be its mode of action. However, by increasing the intracellular pool of Cu binding proteins, it reduces the free Cu pool in blood as effective as chelators and urinary Cu excretion becomes normal. Thus, in addition it would be helpful to measure free Cu in blood for monitoring therapeutic efficacy directly.
A new drug is presently under investigation in clinical trials: bis-choline-tetrathiomolybdate (TTM). It acts as intracellular Cu chelator (84,85). It was shown to improve neurologic symptoms (84). It is also reported to bind free Cu in blood (86). Although the exact mechanism of its action needs more exploration, within hepatocytes it removes Cu bound to proteins and binds it in stable complexes which are cleared into bile (85). At least in high doses it often shows elevation of transaminases indicating cell injury (84-86). Apparently, there is an intracellular redistribution of Cu with concomitant mitochondrial damage (87). This clinical important issue needs attention.
Finally, there is the option of liver transplantation (67). However, it is only chosen in end stage liver disease with poor prognosis due to hepatic failure. In symptomatic Wilson disease the therapeutic goal is to stop progression of liver disease, reversal or improvement of extrahepatic manifestation, and allow an almost normal life expectancy, which in most cases is achievable by the conventional medical therapy (88). Only in some patients, mainly those treated with chelators, neurologic symptoms deteriorate (89). Occasionally disease exacerbation is such severe that patients even become handicapped. The phenomenon is not explained yet. It could be the increase in chelated free Cu, which may enter brain parenchymal cells. Intracellular Cu chelators like TTM or the very old drug British Anti Lewisite (Dimercaprol—painful intramuscular injections!) may theoretically help although it is not systematically investigated in these cases. The metabolic defect in Wilson disease resides in the liver. Correction of the genetic defect would most likely cure the disease. Since gene therapy is still being investigated in two phase I/II clinical trials (39), liver transplantation is established and would at present serve the same purpose. Thus, liver transplantation was suggested also to be applied in patients with a fulminant neurologic course. Indeed, positive outcomes about this approach have been reported (90). However, critics argue since the neurologic disorder is manifested, it is an irreversible damage and also liver transplantation would be of no cure (91).
However, liver transplantation is an unequivocal therapeutic consideration in end stage liver disease which occurs in a cirrhotic liver concomitant with hepatic failure. In Wilson disease it is observed in too late diagnosed and, thus, mainly untreated cases. Liver transplantation also has to be applied in fulminant hepatic failure. It is the rarely observed first manifestation of Wilson disease, mostly in young, predominantly female patients without previous signs of liver disease. It occurs unexpectedly with a very rapid course of deterioration ending in death within a few days. Mechanistically it is an apoptotic process affecting the total liver at once (38). Hepatic failure, coagulopathy, kidney failure and hepatic coma are the sequel of events. In this case liver transplantation with utmost urgency is required. If successful, Wilson disease appears to be history because the genetic defect is corrected and requires no further Cu controlling therapy. The only question which remains open, is whether the mutated ATP7B itself may still induce cell injury in organs other than the liver, e.g., brain.
In addition, several experimental strategies have suggested other possibilities to cure Wilson disease-induced hepatic Cu overload. In one approach, the transduction of an adeno-associated vector serotype 8 (AAV8) encoding the human ATP7B cDNA placed under control of the liver specific α1-antitrypsin promoter allowed long-term correction of copper metabolism in Atp7b null mice (92,93). The gene correction was also associated with significant lowering of cerebral Cu concentrations, most prominently in the cerebellum, cerebellar white matter, corpus callosum, and in some ventricles (94). Another study showed that the blood-brain barrier that complicates the treatment with chelating agents in the setting of Wilson disease can be successfully passed by vectorized liposomes in which chelating agents such as triethylenetetramine (TETA) can be encapsulated. In rats such a strategy resulted in an up to 16-fold higher brain uptake of TETA compared to free TETA (95).
Conclusions
It is established that malfunctional ATP7B is the trigger of liver injury. There is already an exceptional knowledge about the mechanisms by which copper and mutations within the ATP7B gene develop their pathogenic properties during onset and progression of Wilson disease (96,97). Nevertheless, the narrative review presented here shows that all this knowledge is not suitable to prevent the onset of serious symptoms in many cases or to explain the variability in the type and severity of disease-associated symptoms. Furthermore, the efficacy of chelating agents and other therapies are still controversially discussed and there are still many questions unanswered.
In particular, key questions that need to be elucidated are:
- Is it indeed the intracellular free fraction of Cu+ which causes membrane destruction?
- How is the free Cu fraction structurally defined and where exactly is it generated?
- How Cu+ is translocated across intracellular membranes?
In this regard, the following observations are of interest:
- Newborns store excessive Cu without any harm to the liver.
- The years of Cu accumulation in Wilson disease pass without cell injury despite highest Cu levels.
- There are anecdotical reports that despite many years of effective therapy and improvement of liver function, intrahepatic Cu stores remain widely unaffected in comparison to time of diagnosis.
This may feed the hypothesis that it is not just Cu overload but the toxic form of cellular Cu which causes membrane destruction. Then the goal of therapy is (I) to reduce free copper in blood by chelators or zinc and (II) to switch cell toxic Cu into a harmless storage form, e.g., by binding to metallothionein. Whether these are the only protective mechanisms remains open. Uptake in cell organelles, i.e., mitochondria and lysosomes with consequent functional or structural damage requires further exploration.
Limitations of this study
The applied literature research discovered basic findings relevant for pathophysiology of Wilson disease. Key findings were that elevation of free copper in blood is due to impaired biliary excretion in Wilson disease and that this free copper is responsible for copper accumulation in different organs. However, detailed mechanistic descriptions were often missing. Statements of key opinion leaders and commercial driven studies/trials limited the objectivity of published results. In addition, based on the high amount of studies dealing with topics of Wilson disease pathogenesis, it might be possible that several important studies were overseen in this analysis.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal for the series “Liver Diseases: Symptoms, Causes and Novel Therapies”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-22-24/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-22-24/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-24/coif). The series “Liver Diseases: Symptoms, Causes and Novel Therapies” was commissioned by the editorial office without any funding or sponsorship. WS and RW served as unpaid Guest Editors of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wilson Disease: Pathogenesis, Molecular Mechanisms, Diagnosis, Treatment and Monitoring. Weiss KH, Schilsky M. editors. 1. Edition. Academic Press, Elsevier; 2019. ISBN: 978-0-12-811077-5.
- Culotta VC, Gitlin JD. Disorders of copper transport. In: Scriver CR, Beaudet AL, Sly WS, et al. editors. The Molecular and Metabolic Basis of Inherited Disease. New York: McGraw-Hill; 2001:3105-36.
- National Institutes of Health. Office of Dietary Supplements. Copper. Fact sheet for health professionals. Available online: https://ods.od.nih.gov/factsheets/Copper-HealthProfessional/
- Nishito Y, Kambe T. Absorption Mechanisms of Iron, Copper, and Zinc: An Overview. J Nutr Sci Vitaminol (Tokyo) 2018;64:1-7. [Crossref] [PubMed]
- Royer A, Sharman T. Copper Toxicity. 2022 Mar 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557456/
- Dancis A, Yuan DS, Haile D, et al. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell 1994;76:393-402. [Crossref] [PubMed]
- Zhou B, Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci U S A 1997;94:7481-6. [Crossref] [PubMed]
- Kuo YM, Zhou B, Cosco D, et al. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci U S A 2001;98:6836-41. [Crossref] [PubMed]
- Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci U S A 2001;98:6842-7. [Crossref] [PubMed]
- Solioz M, Vulpe C. CPx-type ATPases: a class of P-type ATPases that pump heavy metals. Trends Biochem Sci 1996;21:237-41. [Crossref] [PubMed]
- Larin D, Mekios C, Das K, et al. Characterization of the interaction between the Wilson and Menkes disease proteins and the cytoplasmic copper chaperone, HAH1p. J Biol Chem 1999;274:28497-504. [Crossref] [PubMed]
- Otoikhian A, Barry AN, Mayfield M, et al. Lumenal loop M672-P707 of the Menkes protein (ATP7A) transfers copper to peptidylglycine monooxygenase. J Am Chem Soc 2012;134:10458-68. [Crossref] [PubMed]
- Deschamps P, Kulkarni PP, Gautam-Basak M, et al. The saga of copper(II)–l-histidine. Coord Chem Rev 2005;249:895-909. [Crossref]
- Kirsipuu T, Zadorožnaja A, Smirnova J, et al. Copper(II)-binding equilibria in human blood. Sci Rep 2020;10:5686. [Crossref] [PubMed]
- Haas KL, Putterman AB, White DR, et al. Model peptides provide new insights into the role of histidine residues as potential ligands in human cellular copper acquisition via Ctr1. J Am Chem Soc 2011;133:4427-37. [Crossref] [PubMed]
- Hamza I, Gitlin JD. Copper metabolism and the liver. In: Arias IM, Boyer JL, Chisari FV. editors. The Liver: Biology and Pathology. Philadelphia, Lippincott Williams & Wilkins; 2001:331-43.
- Loudianos G, Gitlin JD. Wilson's disease. Semin Liver Dis 2000;20:353-64. [Crossref] [PubMed]
- Gollan JL, Gollan TJ. Wilson disease in 1998: genetic, diagnostic and therapeutic aspects. J Hepatol 1998;28:28-36. [Crossref] [PubMed]
- Köhn B, Ponnandai Shanmugavel K, Wu M, et al. A Luminal Loop of Wilson Disease Protein Binds Copper and Is Required for Protein Activity. Biophys J 2018;115:1007-18. [Crossref] [PubMed]
- Lutsenko S, LeShane ES, Shinde U. Biochemical basis of regulation of human copper-transporting ATPases. Arch Biochem Biophys 2007;463:134-48. [Crossref] [PubMed]
- Hellman NE, Kono S, Mancini GM, et al. Mechanisms of copper incorporation into human ceruloplasmin. J Biol Chem 2002;277:46632-8. [Crossref] [PubMed]
- Linder MC. Apoceruloplasmin: Abundance, Detection, Formation, and Metabolism. Biomedicines 2021;9:233. [Crossref] [PubMed]
- Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr 2002;22:439-58. [Crossref] [PubMed]
- Palmiter RD. The elusive function of metallothioneins. Proc Natl Acad Sci U S A 1998;95:8428-30. [Crossref] [PubMed]
- Michalska AE, Choo KH. Targeting and germ-line transmission of a null mutation at the metallothionein I and II loci in mouse. Proc Natl Acad Sci U S A 1993;90:8088-92. [Crossref] [PubMed]
- Masters BA, Kelly EJ, Quaife CJ, et al. Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc Natl Acad Sci U S A 1994;91:584-8. [Crossref] [PubMed]
- Schaefer M, Hopkins RG, Failla ML, et al. Hepatocyte-specific localization and copper-dependent trafficking of the Wilson's disease protein in the liver. Am J Physiol 1999;276:G639-46. [PubMed]
- Schaefer M, Roelofsen H, Wolters H, et al. Localization of the Wilson's disease protein in human liver. Gastroenterology 1999;117:1380-5. [Crossref] [PubMed]
- Roelofsen H, Wolters H, Van Luyn MJ, et al. Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology 2000;119:782-93. [Crossref] [PubMed]
- Hamza I, Gitlin JD. Hepatic copper transport. Molecular Pathogenesis of Cholestasis. Trauner M, Jansen P. editors. Berlin: Springer Science & Business Media; 2003:225-34.
- Mukherjee S, Dutta S, Majumdar S, et al. Genetic defects in Indian Wilson disease patients and genotype-phenotype correlation. Parkinsonism Relat Disord 2014;20:75-81. [Crossref] [PubMed]
- Stenson PD, Mort M, Ball EV, et al. The Human Gene Mutation Database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum Genet 2017;136:665-77. [Crossref] [PubMed]
- Parisi S, Polishchuk EV, Allocca S, et al. Characterization of the most frequent ATP7B mutation causing Wilson disease in hepatocytes from patient induced pluripotent stem cells. Sci Rep 2018;8:6247. Erratum in: Sci Rep 2020;10:10476. [Crossref] [PubMed]
- Marchi G, Busti F, Lira Zidanes A, et al. Aceruloplasminemia: A Severe Neurodegenerative Disorder Deserving an Early Diagnosis. Front Neurosci 2019;13:325. [Crossref] [PubMed]
- Fujie T, Segawa Y, Yoshida E, et al. Induction of metallothionein isoforms by copper diethyldithiocarbamate in cultured vascular endothelial cells. J Toxicol Sci 2016;41:225-32. [Crossref] [PubMed]
- Polishchuk EV, Polishchuk RS. The emerging role of lysosomes in copper homeostasis. Metallomics 2016;8:853-62. [Crossref] [PubMed]
- Myers BM, Prendergast FG, Holman R, et al. Alterations in hepatocyte lysosomes in experimental hepatic copper overload in rats. Gastroenterology 1993;105:1814-23. [Crossref] [PubMed]
- Strand S, Hofmann WJ, Grambihler A, et al. Hepatic failure and liver cell damage in acute Wilson's disease involve CD95 (APO-1/Fas) mediated apoptosis. Nat Med 1998;4:588-93. [Crossref] [PubMed]
- European Association for Study of Liver. EASL Clinical Practice Guidelines: Wilson's disease. J Hepatol 2012;56:671-85. [Crossref] [PubMed]
- Attri S, Sharma N, Jahagirdar S, et al. Erythrocyte metabolism and antioxidant status of patients with Wilson disease with hemolytic anemia. Pediatr Res 2006;59:593-7. [Crossref] [PubMed]
- Ortiz JF, Morillo Cox Á, Tambo W, et al. Neurological Manifestations of Wilson's Disease: Pathophysiology and Localization of Each Component. Cureus 2020;12:e11509. [Crossref] [PubMed]
- Litwin T, Dusek P, Szafrański T, et al. Psychiatric manifestations in Wilson's disease: possibilities and difficulties for treatment. Ther Adv Psychopharmacol 2018;8:199-211. [Crossref] [PubMed]
- Dusek P, Litwin T, Członkowska A. Neurologic impairment in Wilson disease. Ann Transl Med 2019;7:S64. [Crossref] [PubMed]
- Dusek P, Bahn E, Litwin T, et al. Brain iron accumulation in Wilson disease: a post mortem 7 Tesla MRI - histopathological study. Neuropathol Appl Neurobiol 2017;43:514-32. [Crossref] [PubMed]
- Dzieżyc-Jaworska K, Litwin T, Członkowska A. Clinical manifestations of Wilson disease in organs other than the liver and brain. Ann Transl Med 2019;7:S62. [Crossref] [PubMed]
- Medici V, LaSalle JM. Genetics and epigenetic factors of Wilson disease. Ann Transl Med 2019;7:S58. [Crossref] [PubMed]
- Lu ZK, Cheng J, Li SM, et al. Phenotypes and ATP7B gene variants in 316 children with Wilson disease. Zhonghua Er Ke Za Zhi 2022;60:317-22. [PubMed]
- Hou H, Chen D, Liu J, et al. Clinical and Genetic Analysis in Neurological Wilson's Disease Patients With Neurological Worsening Following Chelator Therapy. Front Genet 2022;13:875694. [Crossref] [PubMed]
- Fernando M, Hetherington R, Van-Mourik I, et al. Insights into genotype-phenotype correlation of Wilson disease in children. 6th World congress of Paediatric Gastroenterology, Hepatology and Nutrition; Vienna, Austria. Abstracts, June 2021:856-7.
- Arrese M, Ananthananarayanan M, Suchy FJ. Hepatobiliary transport: molecular mechanisms of development and cholestasis. Pediatr Res 1998;44:141-7. [Crossref] [PubMed]
- Gitlin D, Biasucci A. Development of gamma G, gamma A, gamma M, beta IC-beta IA, C 1 esterase inhibitor, ceruloplasmin, transferrin, hemopexin, haptoglobin, fibrinogen, plasminogen, alpha 1-antitrypsin, orosomucoid, beta-lipoprotein, alpha 2-macroglobulin, and prealbumin in the human conceptus. J Clin Invest 1969;48:1433-46. [Crossref] [PubMed]
- Ferenci P, Stremmel W, Członkowska A, et al. Age and Sex but Not ATP7B Genotype Effectively Influence the Clinical Phenotype of Wilson Disease. Hepatology 2019;69:1464-76. [Crossref] [PubMed]
- Litwin T, Gromadzka G, Członkowska A. Gender differences in Wilson's disease. J Neurol Sci 2012;312:31-5. [Crossref] [PubMed]
- Wong RJ, Gish R, Schilsky M, et al. A clinical assessment of Wilson disease in patients with concurrent liver disease. J Clin Gastroenterol 2011;45:267-73. [Crossref] [PubMed]
- Stremmel W, Longerich T, Liere R, et al. Wilson disease - the impact of hyperimmunity on disease activity: A case report. World J Clin Cases 2021;9:1386-93. [Crossref] [PubMed]
- van De Sluis B, Rothuizen J, Pearson PL, et al. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet 2002;11:165-73. [Crossref] [PubMed]
- Gollan JL, Gollan TJ. Wilson disease in 1998: genetic, diagnostic and therapeutic aspects. J Hepatol 1998;28:28-36. [Crossref] [PubMed]
- Loudianos G, Gitlin JD. Wilson's disease. Semin Liver Dis 2000;20:353-64. [Crossref] [PubMed]
- Ferenci P, Zollner G, Trauner M. Hepatic transport systems. J Gastroenterol Hepatol 2002;17:S105-12. [Crossref] [PubMed]
- Gossard AA, Lindor KD. Medical management of chronic cholestatic liver diseases. Can J Gastroenterol 2000;14 Suppl D:93D-8D.
- Pandit A, Bhave S. Present interpretation of the role of copper in Indian childhood cirrhosis. Am J Clin Nutr 1996;63:830S-5S. [Crossref] [PubMed]
- Müller T, Müller W, Feichtinger H. Idiopathic copper toxicosis. Am J Clin Nutr 1998;67:1082S-6S. [Crossref] [PubMed]
- Scheinberg IH, Sternlieb I. Wilson disease and idiopathic copper toxicosis. Am J Clin Nutr 1996;63:842S-5S. [Crossref] [PubMed]
- Woimant F, Djebrani-Oussedik N, Poujois A. New tools for Wilson's disease diagnosis: exchangeable copper fraction. Ann Transl Med 2019;7:S70. [Crossref] [PubMed]
- Martínez-Morillo E, Bauça JMTrace Elements Commission of the Spanish Society of Laboratory Medicine. Biochemical diagnosis of Wilson’s disease: an update. Adv Lab Med 2022;3:103-13. [Crossref]
- Catalani S, Paganelli M, Gilberti ME, et al. Free copper in serum: An analytical challenge and its possible applications. J Trace Elem Med Biol 2018;45:176-80. [Crossref] [PubMed]
- Stremmel W, Weiskirchen R. Therapeutic strategies in Wilson disease: pathophysiology and mode of action. Ann Transl Med 2021;9:732. [Crossref] [PubMed]
- Ferenci P. Review article: diagnosis and current therapy of Wilson's disease. Aliment Pharmacol Ther 2004;19:157-65. [Crossref] [PubMed]
- Ferenci P, Steindl-Munda P, Vogel W, et al. Diagnostic value of quantitative hepatic copper determination in patients with Wilson's Disease. Clin Gastroenterol Hepatol 2005;3:811-8. [Crossref] [PubMed]
- Członkowska A, Litwin T, Dusek P, et al. Wilson disease. Nat Rev Dis Primers 2018;4:21. [Crossref] [PubMed]
- Gao J, Brackley S, Mann JP. The global prevalence of Wilson disease from next-generation sequencing data. Genet Med 2019;21:1155-63. [Crossref] [PubMed]
- Leung M, Aronowitz PB, Medici V. The Present and Future Challenges of Wilson's Disease Diagnosis and Treatment. Clin Liver Dis (Hoboken) 2021;17:267-70. [Crossref] [PubMed]
- Sánchez-Monteagudo A, Ripollés E, Berenguer M, et al. Wilson's Disease: Facing the Challenge of Diagnosing a Rare Disease. Biomedicines. 2021;9:1100. [Crossref] [PubMed]
- Gromadzka G, Chabik G, Mendel T, et al. Middle-aged heterozygous carriers of Wilson's disease do not present with significant phenotypic deviations related to copper metabolism. J Genet 2010;89:463-7. [Crossref] [PubMed]
- Collins CJ, Yi F, Dayuha R, et al. Direct Measurement of ATP7B Peptides Is Highly Effective in the Diagnosis of Wilson Disease. Gastroenterology 2021;160:2367-2382.e1. [Crossref] [PubMed]
- Członkowska A, Rodo M, Wierzchowska-Ciok A, et al. Accuracy of the radioactive copper incorporation test in the diagnosis of Wilson disease. Liver Int 2018;38:1860-6. [Crossref] [PubMed]
- Nudelman-Speckman A, Díaz-Meneses I, Valenzuela M, et al. Evaluation of Wilson Disease With 18F-FDG PET/CT. J Neurol Neurosci 2017;8:223.
- EFSA NDA Panel. (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific opinion on dietary reference values for copper. EFSA J 2015;13:4253. [Crossref]
- McArdle HJ, Kyriakou P, Grimes A, et al. The effect of D-penicillamine on metallothionein mRNA levels and copper distribution in mouse hepatocytes. Chem Biol Interact 1990;75:315-24. [Crossref] [PubMed]
- WALSHE JM. Penicillamine, a new oral therapy for Wilson's disease. Am J Med 1956;21:487-95. [Crossref] [PubMed]
- Walshe JM. Treatment of Wilson's disease with trientine (triethylene tetramine) dihydrochloride. Lancet 1982;1:643-7. [Crossref] [PubMed]
- Suzuki KT, Someya A, Komada Y, et al. Roles of metallothionein in copper homeostasis: responses to Cu-deficient diets in mice. J Inorg Biochem 2002;88:173-82. [Crossref] [PubMed]
- Pérez MJ, Cederbaum AI. Metallothionein 2A induction by zinc protects HEPG2 cells against CYP2E1-dependent toxicity. Free Radic Biol Med 2003;34:443-55. [Crossref] [PubMed]
- Weiss KH, Askari FK, Czlonkowska A, et al. Bis-choline tetrathiomolybdate in patients with Wilson's disease: an open-label, multicentre, phase 2 study. Lancet Gastroenterol Hepatol 2017;2:869-76. [Crossref] [PubMed]
- Stremmel W. Bis-choline Tetrathiomolybdate as Old Drug in a New Design for Wilson's Disease: Good for Brain and Liver? Hepatology 2019;69:901-3. [Crossref] [PubMed]
- Brewer GJ, Askari F, Dick RB, et al. Treatment of Wilson's disease with tetrathiomolybdate: V. Control of free copper by tetrathiomolybdate and a comparison with trientine. Transl Res 2009;154:70-7. [Crossref] [PubMed]
- Kim P, Zhang CC, Thoröe-Boveleth S, et al. Analyzing the Therapeutic Efficacy of Bis-Choline-Tetrathiomolybdate in the Atp7b-/- Copper Overload Mouse Model. Biomedicines 2021;9:1861. [Crossref] [PubMed]
- Stremmel W, Meyerrose KW, Niederau C, et al. Wilson disease: clinical presentation, treatment, and survival. Ann Intern Med 1991;115:720-6. [Crossref] [PubMed]
- Merle U, Schaefer M, Ferenci P, et al. Clinical presentation, diagnosis and long-term outcome of Wilson's disease: a cohort study. Gut 2007;56:115-20. [Crossref] [PubMed]
- Poujois A, Sobesky R, Meissner WG, et al. Liver transplantation as a rescue therapy for severe neurologic forms of Wilson disease. Neurology 2020;94:e2189-202. [Crossref] [PubMed]
- Lankarani KB, Malek-Hosseini SA, Nikeghbalian S, et al. Fourteen Years of Experience of Liver Transplantation for Wilson's Disease; a Report on 107 Cases from Shiraz, Iran. PLoS One 2016;11:e0167890. [Crossref] [PubMed]
- Murillo O, Luqui DM, Gazquez C, et al. Long-term metabolic correction of Wilson's disease in a murine model by gene therapy. J Hepatol 2016;64:419-26. [Crossref] [PubMed]
- Moreno D, Murillo O, Gazquez C, et al. Visualization of the therapeutic efficacy of a gene correction approach in Wilson's disease by laser-ablation inductively coupled mass spectrometry. J Hepatol 2018;68:1088-90. [Crossref] [PubMed]
- Uerlings R, Moreno D, Murillo O, et al. Brain copper storage after genetic long-term correction in a mouse model of Wilson disease. Neurol Genet 2018;4:e243. [Crossref] [PubMed]
- Tremmel R, Uhl P, Helm F, et al. Delivery of Copper-chelating Trientine (TETA) to the central nervous system by surface modified liposomes. Int J Pharm 2016;512:87-95. [Crossref] [PubMed]
- LiverTox: Clinical and research information on drug-induced liver injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Wilson Disease Agents. 2020 Jul 25. Available online: https://pubmed.ncbi.nlm.nih.gov/31644190/ (last assessed 30 August 2022).
- Członkowska A, Litwin T, Dusek P, et al. Wilson disease. Nat Rev Dis Primers 2018;4:21. [Crossref] [PubMed]
Cite this article as: Stremmel W, Weiskirchen R. Wilson disease: more complex than just simply a copper overload condition?—a narrative review. AME Med J 2022;7:26.

