
Adherence of unpublished case reports to the Case Report (CARE) guidelines: a retrospective cross-sectional analysis of 139 case report manuscripts initially submitted to AME medical journals
Introduction
Case reports are valuable literature for a number of reasons. On the one hand, they are detailed descriptions of the medical problems experienced by one or more patients that are written for medical, scientific, or educational purposes (1). Case reports have been shown to play a role in investigating the potential adverse and beneficial effects of treatments and can help identify new diseases, unusual presentations of common diseases, and manifestations of rare diseases (2). For example, our understanding of the relationship between thalidomide and congenital malformations was mostly initially uncovered from case reports (3,4); similarly, due to an important finding in a case report, propranolol was considered as a possible treatment for infants and children with hemangiomas (5). On the other hand, case reports do not provide strong causal evidence compared with other designs, such as analytical observational studies or randomized controlled trials. Therefore, evidence-based medicine categorizes case reports as lower-grade evidence (6). However, case reports represent a separate form of clinical information and serve as a source of hypothesis-generating exploration when they are reported in an accurate, complete, and transparent manner. For example, case reports can suggest hypotheses for future research, guide individualized treatment, and facilitate comparisons of medical education and services across cultures (7,8). Moreover, case reports constitute a significant proportion of medical articles, and the number of published case reports has increased in recent years. The number of case reports published in 2019, 2020, and 2021 indexed on Web of Science was 35,993, 42,297, and 45,812 respectively (Source: Web of Science, October 26, 2022).
The value of case reports relies particularly on their being accurate, complete, and transparent for reporting. Specifically, case reports written in the absence of reporting guidelines are often not sufficiently rigorous to allow for pooled data analysis, to provide a valid basis for clinical study design, or to adequately guide clinical practice (9,10). This is also why in 2011, the Case Report (CARE) guidelines development group was established to formulate a checklist for the better reporting of case reports. The CARE guidelines were published in 2013 in 7 journals in the same year (11-17) and were translated into multiple languages (18). Since its publication, the CARE guidelines have received widespread recognition from authors, journal editors, and reviewers. They are also available on the Enhancing the Quality and Transparency of Health Research (EQUATOR) network website (19) and are recommended on the front page of the website.
However, previous studies have evaluated the reporting quality of published case reports over diverse clinical areas and reported unsatisfactory results (6,20-23). The reasons behind this may be multifaceted. For authors, they may encounter the lack of scientific writing skills and the unsatisfactory communication between colleagues (24), a shortage of opportunities for professional development, limited financial resources for medical writing coursework, and a lack of encouragement to publish in mainstream journals (25-27). For reviewers and editors, it may also be that they are not always as diligent as they should be due to their heavy workload. It is also possible that the responsibilities of editors, authors, and reviewers in this area are not clearly shared or agreed upon. Of note, existing research that has revealed low adherence to CARE guidelines has largely focused on evaluating the final published articles. This makes it difficult to find the main reasons behind unsatisfactory reporting; after all, the reporting of the final published article has contained efforts from authors, reviewers, and editors. This also makes it unclear whether future efforts to promote better reporting of case reports should be more tailored to authors, editors, or reviewers.
Therefore, this study aimed (I) to evaluate authors’ adherence to CARE guidelines in their first drafts submitted to journals and (II) to obtain information helpful in determining whether future efforts should be more focused on author education or editorial and peer-review education by exploring differences between our findings and those from studies that assessed published case reports.
Methods
Eligible case report drafts inclusion
To obtain a sample that was optimally representative and minimize potential selection bias, we selected a total of 23 journals under our company—AME Publishing Company—which are dedicated to the various fields, such as lung cancer and related thoracic diseases, breast surgery, gynecology, and pelvic medicine. Journals that only published case reports and comprehensive medical journals were both included. The draft case reports submitted to these 23 medical journals between January 2019 and March 2020 were retrospectively and consecutively included. Additionally, these journals all required the submission of CARE checklists as supplementary materials along with case reports.
Reporting quality assessment and data extraction
Two reviewers (KPZ and YZ) independently assessed the reporting quality of the included case reports according to the CARE guidelines (11). The third reviewer (FHY) rechecked the accuracy of the reporting quality assessment. All the reviewers were trained to develop a basic understanding of CARE guidelines. Inconsistencies and individual concerns about specific items were discussed and resolved by consensus. Each reviewer had at least 1 year of experience in reviewing case reports as a journal editor.
Each case report was evaluated by the 30 subitems nested in the 13 items of the CARE guidelines under the following scoring criteria: 1= reported or not applicable, and 0= unreported or partially reported. We calculated the completion percentage of included studies for each CARE item.
In addition, region of origin was defined by the institution location of the first author. Indexation was classified into Science Citation Index (SCI)-indexed, PubMed-indexed and nonindexed. Information concerning these two factors was recorded and tabulated by two reviewers (YLC and FHY).
Statistical analysis
Analysis was completed by using Excel (2020 version; Microsoft Corporation, USA) and SPSS statistical software (version 26; IBM Corporation, USA). The reporting quality data are presented as the percentage of unreported or partially reported studies. The categorical data are expressed as the number (percentage) of cases. The quantitative data are presented as median [range]. We compared the adherence to CARE guidelines of the included studies by origin and indexing status with the Kruskal-Wallis test. P<0.05 was considered statistically significant.
Results
Region and journal distribution of eligible case reports
A total of 139 case reports were included in the analysis. Table 1 summarizes the region and journal distribution of included case reports and their reporting. The vast majority of eligible case reports came from Asia (80.6%) and SCI-indexed journals (78.4%). There were no statistical differences in the reporting quality of eligible case reports in terms of geographical region (P=0.207) or journal indexing (P=0.777) (Table 1).
Table 1
| Characteristics | Number (%) | Reported items, median [range] |
|---|---|---|
| Region | ||
| Total | 139 (100.0) | |
| Asia | 112 (80.6) | 25 [12–30] |
| Europe | 16 (11.5) | 25 [20–30] |
| United States | 10 (7.2) | 26 [22–29] |
| Oceania | 1 (0.7) | 26 |
| Journal | ||
| Total | 139 (100.0) | |
| SCI-indexed | 109 (78.4) | 25 [12–30] |
| PubMed-indexed | 5 (3.6) | 25 [22–28] |
| Not indexed | 25 (18.0) | 24 [20–30] |
SCI, Science Citation Index.
Adherence to the CARE guidelines
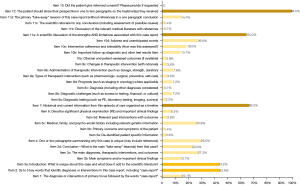
Figure 1 summarizes the adherence to the CARE guidelines of the included 139 case reports. In brief, item 13 (informed consent from the patient) was reported in all eligible case reports. Moreover, most of the articles reported satisfactorily for item 11c (rationale for conclusions), item 11b (discussion of relevant literature), item 8c (diagnosis), and item 5b (primary concerns and symptoms). However, only 2 case reports (1.4%) reported all 30 items required by the CARE guidelines. Specifically, 3 items were the least reported, with more than 50% of articles not reporting or only partially reporting these items: item 12 (patient perspective; 98.6% unsatisfactory), item 7 (timeline; 66.2% unsatisfactory), and item 11a (study strengths and limitations; 64.0% unsatisfactory). Other more frequently underreported items included item 2 (including “case report” in keywords; 43.9% unsatisfactory), item 3a (a unique statement in the abstract; 43.2% unsatisfactory), item 4 (highlight the unique in the introduction; 29.5% unsatisfactory), item 3c (primary diagnosis, therapeutic interventions, and outcome; 27.3% unsatisfactory), item 10d (adverse and unanticipated events; 26.6% unsatisfactory), and item 5c (medical, family, and psychosocial history; 25.9% unsatisfactory).
Discussion
We found that the adherence to the CARE guidelines in the case report drafts was inadequate and that this unsatisfactory reporting was not statistically different across geographic regions or indexed journals. Although case report authors claim adherence to CARE guidelines, only a very few articles reported all 30 subitems of the CARE guidelines. Articles reported unsatisfactorily on many items, with the most worrisome reporting being related to item 12 (patient perspective), item 7 (timeline), and item 11a (strengths and limitations of the study).
Incomplete reporting is a prevalent problem in scientific publications that is present in both in vivo and in vitro studies (28). Chalmers and Glasziou pointed out that at least 50% of research reports are unusable due to incomplete reporting (29). Therefore, journal editors, reviewers, and authors should be encouraged to adhere to reporting guidelines to ensure high quality reporting and reduce waste caused by incomplete or unusable biomedical research reports (30). Moreover, Seguel-Moraga et al. evaluated 201 published case reports on dental health from 2008 to 2018 and clearly identified that these articles did not properly adhere to the CARE guidelines, with item 7 (timeline) item 12 (patient perspective), and item 13 (informed consent) being the 3 least frequently reported items (22). Park et al. evaluated 827 published case reports and identified a lack of reporting for critical items in the CARE guidelines, including item 8b (diagnostic challenges), item 12 (patient perspective), item 13 (informed consent), item 10c (intervention compliance and tolerability), and item 10d (adverse events) (31).
The results of these studies evaluating published case reports and our results evaluating unpublished first drafts both suggest suboptimal adherence to CARE guidelines for case reports, indicating a room for greater efforts by authors, editors, and reviewers alike. There are a number of overlapping findings between our studies and those assessing published case reports: item 12 (patient perspective), item 7 (timeline), and item 10d (adverse and unanticipated events) were all noted as seriously incomplete or unreported. This suggests a continuing need for significant improvement in the quality of reporting of patient perspectives, timelines, and adverse and unintended events, both in the original draft and in the final publication. It also indicates that authors are not the only ones underperforming in these areas and that editors and reviewers also need to be more attentive here. In terms of the areas of concern (i.e., items 12, 7, and 10d), there is much room for future efforts in author, editorial, and reviewer education.
Along with the results from overlapping, new findings emerged from our evaluation of the first draft of case reports that were not found in previous studies evaluating published case reports (6,20,31): item 11a (strengths and limitation discussion), item 2 (the inclusion of “case report” in keywords), and item 3a (unique statement in the abstract) were all underreported in our analysis. This discrepancy might have arisen due to our different definition of “unreported” or from the fact that studies differed in the specialty areas and time periods in which case reports were included. Most importantly, the case reports we included were drafts submitted to journals, which is the biggest difference between this study and those previous. Therefore, the poor adherence of item 11a may reflect the overly optimistic statements of authors concerning the academic value of their research in order to receive positive comments from editors and reviewers. The underperformance of item 2 may be due to the fact that the authors only considered technical terms regarding the study topic and ignored the study type when formulating keywords. The inadequate performance of item 3a may be a result of the uniqueness of the case report not being evident or the authors’ poor writing skills. Additionally, these three items were infrequently mentioned in previous studies assessing published case reports, suggesting that editors and reviewers might have had greater success working in these areas. These results suggest that items 11a, 2, and 3a may be topics of focus in future author education.
Of further note, although our results found no statistical difference in geographic region for poor adherence, our included articles were predominantly from Asia. Due to the larger sample size in Asia, our findings might be more applicable to Asian authors. With the CARE guidelines being listed on the front page of the EQUATOR website, with a very detailed explanation and elaboration file (32), and with author instructions in the AME Publishing Company journals that clearly state that case reports should follow CARE guidelines (33), the remaining poor CARE compliance of case report drafts indicates the need for additional educational approaches and platforms, such as the use of multiple social media platforms, more online and offline training, and the integration of case report reporting into postgraduate courses.
The main strength of this study is the evaluation of adherence to CARE guidelines for case report drafts that have not been published. To the best of our knowledge, no similar studies have been conducted. However, the present study has some limitations. First, it employed a retrospective design, and thus it may be necessary to conduct prospective studies to analyze the quality case report drafts received in the future to determine if there is any improvement in CARE guideline adherence by the authors. Second, the proportion of articles included in this study outside of Asia was too low. More case report drafts from outside of Asia need to be included in future studies.
Conclusions
The adherence of case reports to CARE guidelines remains poor, both in drafts just submitted to journals that primarily reflect the efforts of authors and in formally published case reports that reflect the additional efforts of editors and reviewers. Future tailored educational strategies are recommended based on the performance of authors, editors, and reviewers on different items of the CARE guidelines.
Acknowledgments
We acknowledge Yao Zhu for her assistance in data collection and John Gray for polishing the language.
Funding: None.
Footnote
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-22-94/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-22-94/coif). All authors declare that they are full-time employees of AME Publishing Company (publisher of AME Medical Journal). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Porta M. A Dictionary of Epidemiology. 6th ed. New York: Oxford University Press, 2014:376.
- Hauben M, Aronson JK. Gold standards in pharmacovigilance: the use of definitive anecdotal reports of adverse drug reactions as pure gold and high-grade ore. Drug Saf 2007;30:645-55. [Crossref] [PubMed]
- Lenz W, Pfeiffer RA, Kosenow W, et al. Thalidomide and congenital abnormalities. Lancet 1962;279:45-6.
- Speirs AL. Thalidomide and congenital abnormalities. Lancet 1962;1:303-5. [Crossref] [PubMed]
- Sans V, de la Roque ED, Berge J, et al. Propranolol for severe infantile hemangiomas: follow-up report. Pediatrics 2009;124:e423-31. [Crossref] [PubMed]
- Sun GH, Aliu O, Hayward RA. Open-access electronic case report journals: the rationale for case report guidelines. J Clin Epidemiol 2013;66:1065-70. [Crossref] [PubMed]
- Jenicek M. Clinical case reporting in evidence-based medicine. New York: Oxford University Press, 2001.
- Riley D. Case reports in the era of clinical trials. Glob Adv Health Med 2013;2:10-1. [Crossref] [PubMed]
- Kljakovic M. Single cases in general practice and general medical journals. Aust Fam Physician 2002;31:669-73.
- Kaszkin-Bettag M, Hildebrandt W. Case reports on cancer therapies: the urgent need to improve the reporting quality. Glob Adv Health Med 2012;1:8-10. [Crossref] [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep 2013;2013:bcr2013201554. [Crossref] [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: Consensus-based Clinical Case Reporting Guideline Development. Glob Adv Health Med 2013;2:38-43. [Crossref] [PubMed]
- Gagnier JJ, Riley D, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Dtsch Arztebl Int 2013;110:603-8. [Crossref] [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol 2014;67:46-51. [Crossref] [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. J Med Case Rep 2013;7:223. [Crossref] [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case report guideline development. J Diet Suppl 2013;10:381-90. [Crossref] [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013;53:1541-7. [Crossref] [PubMed]
- Scientific Writing in Health and Medicine (SWIHM). Resources. Available online: https://swihm.com/resources/
- Simera I, Altman DG, Moher D, et al. Guidelines for reporting health research: the EQUATOR network's survey of guideline authors. PLoS Med 2008;5:e139. [Crossref] [PubMed]
- Eldawlatly A, Alsultan D, Al Dammas F, et al. Adaptation of CARE (CAse REport) guidelines on published case reports in the Saudi Journal of Anesthesia. Saudi J Anaesth 2018;12:446-9. [Crossref] [PubMed]
- Dragnev NC, Wong SL. Do we CARE about the quality of case reports? A systematic assessment. J Surg Res 2018;231:428-33. [Crossref] [PubMed]
- Seguel-Moraga P, Onetto JE, E, Uribe S. Reporting quality of case reports about dental trauma published in international journals 2008-2018 assessed by CARE guidelines. Dent Traumatol 2021;37:345-53. [Crossref] [PubMed]
- Freer R, Rowett A, Camm CF. An assessment of adherence to CARE reporting standards by case reports published in European Heart Journal - Case Reports in 2018. Eur Heart J Case Rep 2020;4:1-5. [Crossref] [PubMed]
- Rawson RE, Quinlan KM, Cooper BJ, et al. Writing-skills development in the health professions. Teach Learn Med 2005;17:233-8. [Crossref] [PubMed]
- Griffin MF, Hindocha S. Publication practices of medical students at British medical schools: experience, attitudes and barriers to publish. Med Teach 2011;33:e1-8. [Crossref] [PubMed]
- Derish PA, Maa J, Ascher NL, et al. Enhancing the mission of academic surgery by promoting scientific writing skills. J Surg Res 2007;140:177-83. [Crossref] [PubMed]
- Marusić A, Marusić M. Teaching students how to read and write science: a mandatory course on scientific research and communication in medicine. Acad Med 2003;78:1235-9. [Crossref] [PubMed]
- Vandenbroucke JP. In defense of case reports and case series. Ann Intern Med 2001;134:330-4. [Crossref] [PubMed]
- Glasziou P, Altman DG, Bossuyt P, et al. Reducing waste from incomplete or unusable reports of biomedical research. Lancet 2014;383:267-76. [Crossref] [PubMed]
- Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet 2009;374:86-9. [Crossref] [PubMed]
- Park JH, Lee S, Kim TH, et al. Current status of case reports and case series reported by Korean Medicine doctors in primary clinics: A systematic review. Integr Med Res 2020;9:100417. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- AME Medical Journal. Guidelines for Authors. 2.5.1 Reporting Checklist. Available online: https://amj.amegroups.com/pages/view/guidelines-for-authors#content-2-5-1
Cite this article as: Yang F, Cheng Y, Zhang K. Adherence of unpublished case reports to the Case Report (CARE) guidelines: a retrospective cross-sectional analysis of 139 case report manuscripts initially submitted to AME medical journals. AME Med J 2022;7:36.

