
Risk factors for 5-day bleeding after endoscopic treatments for gastroesophageal varices in liver cirrhosis
Introduction
Gastroesophageal varices are one of the most common complications of liver cirrhosis (1-3). Variceal bleeding can result in a high risk of death, especially in patients with Child-Pugh class C (2,3). Therapeutic modalities for gastroesophageal varices have been greatly improved (4-7). Currently, endoscopic treatment is the first-line choice for the treatment of acute variceal bleeding and prevention of variceal rebleeding and first bleeding of high-risk varices (8-10). However, a proportion of patients will develop the bleeding after endoscopic treatment (11). In a retrospective cohort of 174 patients, the 1-month, 1-year, and 5-year cumulative rebleeding rate was 10.2%, 30.0%, and 51.0% in patients emergently hospitalized for esophageal variceal bleeding, respectively (11). Early recognition of such a bleeding risk after endoscopic treatments, especially during hospitalization, is very important for both patients and physicians. First, if a cirrhotic patient had some potential risk factors for developing the early bleeding after endoscopic treatments, the physicians should correct them before endoscopic treatments. Second, since the introduction of covered stents, transjugular intrahepatic portosystemic shunts (TIPS) have a low shunt dysfunction (12). Additionally, recent meta-analysis confirmed that covered TIPS should be more effective than endoscopic treatment for the prevention of variceal rebleeding (13). Thus, as for cirrhotic patient at a high risk for developing early bleeding after endoscopic treatments, endoscopic treatments might be inappropriate and covered TIPS would be further considered.
Herein, we conducted a retrospective study to evaluate the risk factors for 5-day bleeding after endoscopic treatments for gastroesophageal varices in liver cirrhosis.
Methods
In this retrospective study, we screened all cirrhotic patients who were admitted to our department between January and March 2016 and underwent endoscopic treatments for gastroesophageal varices. Malignancy, such as confirmed diagnosis of hepatocellular carcinoma or suspected diagnosis of liver cancer, was not excluded. Before endoscopic procedures, all patients should sign the written informed consents. The relevant data at patients’ admissions were collected as follows: age, sex, etiology of liver cirrhosis, prior endoscopic treatment, and laboratory data (e.g., hemoglobin, red blood cell, white blood cell, platelets count, total bilirubin, albumin, creatinine, blood urea nitrogen, prothrombin time, INR, activated partial thromboplastin time, D-dimer, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyltransferase, potassium, and sodium). Additionally, we collected the type and number of endoscopic treatments at their admissions, use of proton pump inhibitors and somatostatin and its analogs drugs after endoscopic treatments, 5-day bleeding after endoscopic treatments, and in-hospital death. Five-day bleeding is an important parameter for the management of variceal bleeding (4-7), which is defined as the development of bleeding within 5 days from endoscopic treatments. The study protocol was approved by the Medical Ethics Committee of General Hospital of Shenyang Military Area. Approval number was No. k(2017)9. Informed written patient consents for this study were waived due to the retrospective nature.
At our department, endoscopic treatments were performed by two endoscopists (X Shao and X Guo) with the assistance of one nurse. The type of endoscopic treatments for gastroesophageal varices included endoscopic variceal ligation (EVL), endoscopic injection sclerotherapy (EIS), and glue injection. The type of endoscopic treatments was primarily dependent upon the type of gastroesophageal varices and endoscopists’ choices.
All statistical analyses were performed by SPSS statistics version 17.0.0. Categorical and continuous data were presented as frequency (percentage) and mean ± standard deviation and median (range), respectively. Categorical and continuous data between two groups were compared by Chi-square tests and non-parametric Mann-Whitney U tests, respectively. Receiver operating curve (ROC) analysis was performed to explore the diagnostic accuracy of relevant variables. The area under curve (AUC) with 95% confidence interval (CI) was calculated. A best cut-off value of any significant variable with a sensitivity and a specificity was also calculated. Two-sided P<0.05 was considered to be statistically significant.
Results
In total, 95 patients were included in the study (Table 1). Among them, 16 patients had undergone at least one endoscopic treatment. Three patients had two endoscopic treatments at the same admission, and 92 patients had only one endoscopic treatment at the same admission. Type of endoscopic treatments during their admissions included EVL alone (n=83), EIS alone (n=3), glue injection alone (n=6), EVL in combination glue injection (n=2), and EVL in combination with EIS (n=1).
Table 1
| Variables | No. pts available | Mean ± SD or frequency (percentage) | Median (range) |
|---|---|---|---|
| Age (years) | 95 | 57.81±12.19 | 58.58 (27.24–82.74) |
| Sex (male/female) | 95 | 48 (50.5%)/47 (49.5%) | |
| Etiology | 95 | ||
| HBV | 31 (32.6%) | ||
| HCV | 10 (10.5%) | ||
| Alcohol | 12 (12.6%) | ||
| HBV + alcohol | 6 (6.3%) | ||
| HCV + alcohol | 1 (1.1%) | ||
| Drug | 2 (2.1%) | ||
| Autoimmunity | 5 (5.3%) | ||
| Unknown or others | 28 (29.5%) | ||
| HCC | 95 | 8 (8.4%) | |
| Prior endoscopic treatment | 95 | 16 (16.8%) | |
| RBC (1012/L) | 95 | 3.22±0.77 | 3.18 (1.91–5.86) |
| Hb (g/L) | 95 | 88.68±25.24 | 84 [44–192] |
| WBC (109/L) | 95 | 4.43±2.75 | 3.9 [1–17] |
| PLT (109/L) | 95 | 94.75±69.49 | 81 [23–445] |
| TBIL (μmol/L) | 94 | 24.04±16.29 | 20.4 (7.9–95.8) |
| ALB (g/L) | 93 | 31.09±6.26 | 30.4 (17.8-51.3) |
| ALT (U/L) | 94 | 25.84±19.03 | 20.31 (1.98–118.66) |
| AST (U/L) | 94 | 38.07±28.44 | 28.33 (11.26–180.19) |
| ALP (U/L) | 94 | 102.41±73.37 | 80.72 (25–494.42) |
| GGT (U/L) | 94 | 76.59±147.25 | 28.06 (9.76–1027.57) |
| BUN (mmol/L) | 94 | 6.51±4.26 | 5.53 (1.89–28.33) |
| Cr (mmol/L) | 94 | 67.53±23.37 | 63.30 (37.12–167.35) |
| K (mmol/L) | 94 | 3.90±0.54 | 3.84 (2.86–5.95) |
| Na (mmol/L) | 94 | 138.74±3.78 | 139 (129.7–152.9) |
| PT (s) | 93 | 16.19±2.55 | 16.19 (11.5–25.60) |
| INR | 93 | 1.32±0.27 | 1.27 (0.87–2.46) |
| APTT (s) | 93 | 38.38±4.57 | 37.7 (29.2–50.50) |
| D-dimer (mg/L) | 65 | 1.68±1.65 | 1.09 (0.23–10.34) |
| Ammonia (μmol/L) | 52 | 50.27±39.94 | 36.50 (7.04–236) |
| MELD score | 94 | 6.63±5.10 | 5.74 (−3.15 to 22.32) |
| Drugs | 95 | ||
| Esomeprazole | 89 (93.7%) | ||
| Pantoprazole | 2 (2.1%) | ||
| Somatostatin | 37 (38.9%) | ||
| Octreotide | 54 (56.8%) | ||
| Endoscopic treatment | 95 | ||
| EVL | 83 (87.4%) | ||
| EIS | 3 (3.2%) | ||
| Glue injection | 6 (6.3%) | ||
| EVL + Glue injection | 2 (2.1%) | ||
| EVL + EIS | 1 (1.1%) | ||
| Length of hospitalization (days) | 95 | 11.40±4.85 | 10 [4–35] |
| 5-day bleeding after endoscopic treatments | 95 | 8 (8.4%) | |
| In-hospital death | 95 | 2 (2.1%) |
HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; RBC, red blood cell; Hb, hemoglobin; WBC, white blood cell; PLT, platelet; TBIL, total bilirubin; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; BUN, blood urea nitrogen; Cr, creatinine; K, potassium; Na, sodium; PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time; MELD, model for end stage liver disease; EVL, endoscopic variceal ligation; EIS, endoscopic injection sclerotherapy.
Eight (8.6%) patients developed 5-day bleeding after endoscopic treatments. Compared with those who did not develop bleeding, patients who developed bleeding had significantly lower albumin levels at the baseline, higher prothrombin time, INR, and D-dimer level at the baseline, longer duration of hospitalizations, and higher in-hospital mortality (Table 2). Notably, the MELD score was not significantly different between the two groups.
Table 2
| Variables | Bleeding (n=8) | No bleeding (n=87) | P value | |||
|---|---|---|---|---|---|---|
| No. pts available | Mean ± SD or frequency (percentage) | No. pts available | Mean ± SD or frequency (percentage) | |||
| Age (years) | 8 | 63.95±10.61 | 87 | 57.24±12.22 | 0.167 | |
| Sex (male/female) | 8 | 2 (25%)/6 (75%) | 87 | 46 (52.9%)/41 (47.1%) | 0.131 | |
| Etiology | 8 | 87 | 0.375 | |||
| HBV | 2 (25%) | 29 (33.3%) | ||||
| HCV | 2 (25%) | 8 (9.2%) | ||||
| Alcohol | 1 (12.5%) | 11 (12.6%) | ||||
| HBV + alcohol | 0 (0%) | 6 (6.9%) | ||||
| HCV + alcohol | 0 (0%) | 1 (1.1%) | ||||
| Drug | 1 (12.5%) | 1 (1.1%) | ||||
| Autoimmunity | 0 (0%) | 5 (5.7%) | ||||
| Unknown or others | 2 (25%) | 26 (29.9%) | ||||
| HCC | 8 | 0 (0%) | 87 | 8 (9.2%) | 0.370 | |
| Prior endoscopic treatment | 8 | 2 (25%) | 87 | 14 (16.1%) | 0.519 | |
| RBC (1012/L) | 8 | 2.75±0.27 | 87 | 3.26±0.78 | 0.062 | |
| Hb (g/L) | 8 | 76.63±9.68 | 87 | 89.79±25.96 | 0.155 | |
| WBC (109/L) | 8 | 5.89±2.89 | 87 | 4.29±2.71 | 0.060 | |
| PLT (109/L) | 8 | 74.88±25.08 | 87 | 99.8±76.92 | 0.639 | |
| TBIL (μmol/L) | 8 | 39.1±30.85 | 86 | 22.64±13.74 | 0.074 | |
| ALB (g/L) | 8 | 26.01±5.60 | 85 | 31.57±6.13 | 0.020 | |
| ALT (U/L) | 8 | 26.50±37.51 | 86 | 25.78±16.75 | 0.101 | |
| AST (U/L) | 8 | 48.52±54.16 | 86 | 37.10±25.14 | 0.924 | |
| ALP (U/L) | 8 | 73.17±32.81 | 86 | 105.13±75.59 | 0.145 | |
| GGT (U/L) | 8 | 26.46±18.98 | 86 | 81.25±153.08 | 0.124 | |
| BUN (mmol/L) | 8 | 8.16±4.64 | 86 | 6.36±4.22 | 0.107 | |
| Cr (mmol/L) | 8 | 57.90±16.35 | 86 | 68.42±23.79 | 0.203 | |
| K (mmol/L) | 8 | 3.92±0.69 | 86 | 3.90±0.53 | 0.876 | |
| Na (mmol/L) | 8 | 139.83±7.07 | 86 | 138.64±3.38 | 0.436 | |
| PT (s) | 8 | 19.20±2.79 | 85 | 15.90±2.36 | 0.001 | |
| INR | 8 | 1.61±0.38 | 85 | 1.30±0.24 | 0.007 | |
| APTT (s) | 8 | 39.1±5.56 | 85 | 38.32±4.51 | 0.547 | |
| D-dimer (mg/L) | 8 | 2.68±0.98 | 57 | 1.54±1.68 | 0.002 | |
| Ammonia (μmol/L) | 6 | 39.84±22.38 | 46 | 51.63±41.66 | 0.731 | |
| MELD score | 8 | 9.03±6.14 | 86 | 6.41±4.97 | 0.297 | |
| Drugs | 8 | 87 | ||||
| Esomeprazole | 8 (100%) | 81 (93.1%) | 0.443 | |||
| Pantoprazole | 0 (0%) | 2 (2.3%) | 0.665 | |||
| Somatostatin | 4 (50%) | 33 (37.9%) | 0.503 | |||
| Octreotide | 4 (50%) | 50 (57.5%) | 0.683 | |||
| Endoscopic treatment | 8 | 87 | 0.498 | |||
| EVL | 6 (75%) | 77 (88.5%) | ||||
| EIS | 1 (12.5%) | 2 (2.3%) | ||||
| Glue injection | 1 (12.5%) | 5 (5.7%) | ||||
| EVL + Glue injection | 0 (0%) | 2 (2.3%) | ||||
| EVL + EIS | 0 (0%) | 1 (1.1%) | ||||
| Length of hospitalization (days) | 8 | 18.63±9.40 | 87 | 10.74±3.63 | 0.011 | |
| In-hospital death | 8 | 2 (25%) | 87 | 0 (0%) | 0.006 | |
HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; RBC, red blood cell; Hb, hemoglobin; WBC, white blood cell; PLT, platelet; TBIL, total bilirubin; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; BUN, blood urea nitrogen; Cr, creatinine; K, potassium; Na, sodium; PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time; MELD, model for end stage liver disease; EVL, endoscopic variceal ligation; EIS, endoscopic injection sclerotherapy.
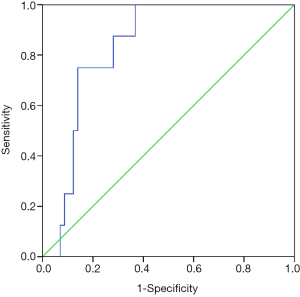
In the ROC analysis (Figure 1), the AUC of albumin level for predicting the risk of being free of 5-day bleeding was 0.750 (95% CI: 0.571–0.929, P=0.020). The best cut-off value of albumin level was 28.05 g/L with a sensitivity of 74.1% and a specificity of 75%.
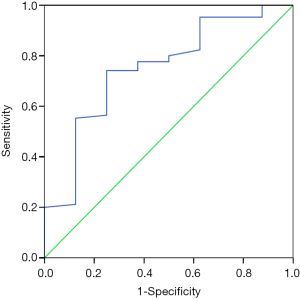
In the ROC analysis (Figure 2), the AUC of prothrombin time for predicting the risk of 5-day bleeding was 0.850 (95% CI: 0.761–0.939, P=0.001). The best cut-off value of prothrombin time was 16.65 seconds with a sensitivity of 100% and a specificity of 69.4%.
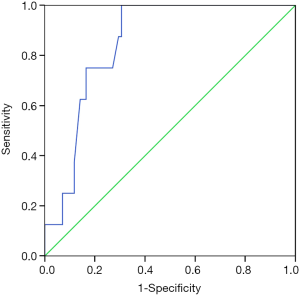
In the ROC analysis (Figure 3), the AUC of INR for predicting the risk of 5-day bleeding was 0.790 (95% CI: 0.661–0.918, P=0.007). The best cut-off value of INR was 1.335 with a sensitivity of 87.5% and a specificity of 69.4%.
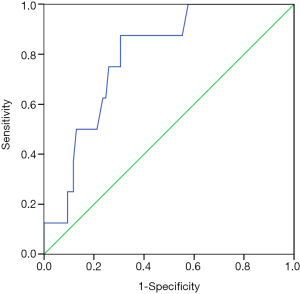
In the ROC analysis (Figure 4), the AUC of D-dimer level for predicting the risk of 5-day bleeding was 0.833 (95% CI: 0.729–0.938, P=0.002). The best cut-off value of D-dimer level was 1.175 mg/L with a sensitivity of 100% and a specificity of 63.2%.
Only two patients died during their hospitalization. Therefore, we could not analyze the risk factors associated with in-hospital death.
Discussion
Our study showed that patients who developed 5-day bleeding after endoscopic treatments had significantly longer lengths of hospitalization and higher in-hospital mortality than patients who did not develop. These findings suggested the importance of avoiding the occurrence of 5-day bleeding after endoscopic treatments and prompted us to identify the patients at a high risk of developing 5-day bleeding. Albumin, prothrombin time, INR, and D-dimer level were significantly associated with the 5-day bleeding risk after endoscopic therapy. Notably, our study did not find that MELD score was a significant risk factor. MELD score was composed of total bilirubin, creatinine, and INR. Indeed, the first two components (i.e., total bilirubin and creatinine) were not associated with the 5-day bleeding risk. Certainly, there was a potential bias in the selection of patients before endoscopic treatments. At our study, the maximum TBIL level of our patients was 95.8 µmol/L, and the maximum creatinine level of our patients was 167.35 mmol/L. If the patients had a very high total bilirubin or creatinine level, they would be excluded from endoscopic treatments.
Prothrombin time and INR are traditional coagulation tests in our clinical practice. Current evidence is very controversial regarding the role of prothrombin time and INR in assessing the bleeding risk in liver cirrhosis (14). The opponents thought that liver cirrhosis had a risk of both thrombotic and bleeding states (15), and that prothrombin time and INR could not globally reflect the balance between them. By contrast, the supporters believed that prothrombin time and INR not only reflected the coagulation profile, but also indicated the severity of liver dysfunction in liver diseases. Recently, Hshieh et al. conducted a retrospective case-control study to evaluate the association of INR with bleeding risk in cirrhotic patients with esophageal varices (16). A total of 74 cases with bleeding esophageal varices and 74 controls with a history of non-bleeding esophageal varices were included. Case group had a significantly lower mean INR at presentation than control group (1.61 vs. 1.74, P=0.03). Notably, 19% (14/74) of the cases failed to control bleeding, and the failure to control bleeding was significantly associated with a higher median INR (1.8 vs. 1.5, P=0.02). These findings seemed to be consistent with ours.
D-dimer level is a laboratory parameter reflecting the fibrinolysis state. A low D-dimer level can be employed for the exclusion of the venous thromboembolism (17). Our previous study also confirmed the association of D-dimer level with the severity of liver dysfunction and in-hospital mortality in liver cirrhosis, regardless of etiology and major clinical presentations (18). In the present study, we further found a significant association between D-dimer and 5-day bleeding risk after endoscopic therapy. Unfortunately, D-dimer is not regularly screened in all patients (about 60% of patients underwent the D-dimer tests). Thus, well-designed prospective studies should be warranted to confirm this finding.
The role of proton pump inhibitor therapy in the prevention of bleeding after endoscopic therapy has been explored. In 2005, Shaheen et al. performed a randomized controlled trial and found that pantoprazole reduced the size of ulcers in patients who underwent VBL (19). But the total number of ulcers and other outcomes were similar between patients who underwent EVL and those who did not. On the basis of this study, the current UK guideline did not recommend any proton pump inhibitor therapy for the management of variceal bleeding (20). By contrast, in 2012, Hidaka et al. performed another randomized controlled trial and found that the long-term administration of rabeprazole reduced the treatment failure after EVL (21). More recently, Kang et al. retrospectively analyzed the risk factors associated with early post-EVL bleeding and found that proton pump inhibitor therapy significantly decreased the incidence of early post-EVL bleeding (22). At our department, proton pump inhibitor therapy was regularly given after endoscopic therapy in all but two patients. And only esomeprazole or pantoprazole was selected in our study.
The limitations of this study were as follows. First, the study population was a little heterogeneous according to the occurrence and prior therapy of variceal bleeding. Some of our patients had a history of variceal bleeding and underwent endoscopic treatments. However, we found that prior endoscopic treatments were not significantly associated with the risk of 5-day bleeding. Second, the data were retrospectively collected, although we thoroughly reviewed the records of our endoscopic treatments. Not all patients had complete laboratory data. Third, the long-term follow-up outcomes were not evaluated. Fourth, we planned to perform the statistical analyses according to the type of endoscopic treatments. However, a majority of patients underwent EVL alone; by contrast, only a minority of patients underwent EIS alone (n=3), glue injection alone (n=6), or a combination therapy (n=3). Thus, a subgroup analysis might be available. Fifth, the present study did not explore the influence of portal vein thrombosis on the prognosis of cirrhotic patients (23-26), because not all patients underwent contrast-enhanced CT or ultrasound of portal vein patency. An ongoing prospective study at our department will explore this issue (ClinicalTrials.gov Identifier: NCT02335580).
In conclusion, we found that albumin, prothrombin time, INR, and D-dimer were four important risk factors associated with 5-day bleeding after endoscopic treatments in liver cirrhosis. Future studies should attempt to resolve how to decrease the risk of 5-day bleeding by improving the four clinical parameters. Additionally, whether patients at high risks for 5-day bleeding should directly undergo covered TIPS needs to be further explored.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.03.03). This work was partially presented as a poster in the 26th Conference of the APASL Annual Meeting in Shanghai, China. Xingshun Qi serves as an Editor-in-Chief of AME Medical Journal. The other authors have no conflicts of interest to declare.
Ethical Statement: Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Medical Ethics Committee of General Hospital of Shenyang Military Area. Approval number was No. k(2017)9. Informed written patient consents for this study were waived due to the retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383:1749-61. [Crossref] [PubMed]
- Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 2010;362:823-32. [Crossref] [PubMed]
- Sharara AI, Rockey DC. Gastroesophageal variceal hemorrhage. N Engl J Med 2001;345:669-81. [Crossref] [PubMed]
- de Franchis R. Updating consensus in portal hypertension: report of the Baveno III Consensus Workshop on definitions, methodology and therapeutic strategies in portal hypertension. J Hepatol 2000;33:846-52. [Crossref] [PubMed]
- de Franchis R. Evolving consensus in portal hypertension. Report of the Baveno IV consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2005;43:167-76. [Crossref] [PubMed]
- de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol 2010;53:762-8. [Crossref] [PubMed]
- de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;63:743-52. [Crossref] [PubMed]
- Hwang JH, Shergill AK, Acosta RD, et al. The role of endoscopy in the management of variceal hemorrhage. Gastrointest Endosc 2014;80:221-7. [Crossref] [PubMed]
- Qureshi W, Adler DG, Davila R, et al. ASGE Guideline: the role of endoscopy in the management of variceal hemorrhage, updated July 2005. Gastrointest Endosc 2005;62:651-5. [Crossref] [PubMed]
- Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46:922-38. [Crossref] [PubMed]
- Cho H, Nagata N, Shimbo T, et al. Recurrence and prognosis of patients emergently hospitalized for acute esophageal variceal bleeding: A long-term cohort study. Hepatol Res 2016;46:1338-46. [Crossref] [PubMed]
- Qi X, Tian Y, Zhang W, et al. Covered versus bare stents for transjugular intrahepatic portosystemic shunt: an updated meta-analysis of randomized controlled trials. Therap Adv Gastroenterol 2017;10:32-41. [Crossref] [PubMed]
- Qi X, Tian Y, Zhang W, et al. Covered TIPS for secondary prophylaxis of variceal bleeding in liver cirrhosis: A systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2016;95:e5680. [Crossref] [PubMed]
- Li J, Qi X, Deng H, et al. Association of conventional haemostasis and coagulation tests with the risk of acute upper gastrointestinal bleeding in liver cirrhosis: a retrospective study. Gastroenterol Rep (Oxf) 2016;4:315-9. [PubMed]
- Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011;365:147-56. [Crossref] [PubMed]
- Hshieh TT, Kaung A, Hussain S, et al. The international normalized ratio does not reflect bleeding risk in esophageal variceal hemorrhage. Saudi J Gastroenterol 2015;21:254-8. [Crossref] [PubMed]
- Geersing GJ, Janssen KJ, Oudega R, et al. Excluding venous thromboembolism using point of care D-dimer tests in outpatients: a diagnostic meta-analysis. BMJ 2009;339:b2990. [Crossref] [PubMed]
- Li Y, Qi X, Li H, et al. D-dimer level for predicting the in-hospital mortality in liver cirrhosis: A retrospective study. Exp Ther Med 2017;13:285-9. [PubMed]
- Shaheen NJ, Stuart E, Schmitz SM, et al. Pantoprazole reduces the size of postbanding ulcers after variceal band ligation: a randomized, controlled trial. Hepatology 2005;41:588-94. [Crossref] [PubMed]
- Tripathi D, Stanley AJ, Hayes PC, et al. UK guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015;64:1680-704. [Crossref] [PubMed]
- Hidaka H, Nakazawa T, Wang G, et al. Long-term administration of PPI reduces treatment failures after esophageal variceal band ligation: a randomized, controlled trial. J Gastroenterol 2012;47:118-26. [Crossref] [PubMed]
- Kang SH, Yim HJ, Kim SY, et al. Proton Pump Inhibitor Therapy Is Associated With Reduction of Early Bleeding Risk After Prophylactic Endoscopic Variceal Band Ligation: A Retrospective Cohort Study. Medicine (Baltimore) 2016;95:e2903. [Crossref] [PubMed]
- Qi X, Bai M, Yang Z, et al. Occlusive portal vein thrombosis as a new marker of decompensated cirrhosis. Med Hypotheses 2011;76:522-6. [Crossref] [PubMed]
- Qi X, Dai J, Jia J, et al. Association between portal vein thrombosis and survival of liver transplant recipients: a systematic review and meta-analysis of observational studies. J Gastrointestin Liver Dis 2015;24:51-9, 4 p following 59.
- Qi X, Dai J, Yang M, et al. Association between Portal Vein Thrombosis and Survival in Non-Liver-Transplant Patients with Liver Cirrhosis: A Systematic Review of the Literature. Gastroenterol Res Pract 2015;2015:480842.
- Qi X, Su C, Ren W, et al. Association between portal vein thrombosis and risk of bleeding in liver cirrhosis: A systematic review of the literature. Clin Res Hepatol Gastroenterol 2015;39:683-91. [Crossref] [PubMed]
Cite this article as: Sun R, Qi X, Zou D, Shao X, Li H, Guo X. Risk factors for 5-day bleeding after endoscopic treatments for gastroesophageal varices in liver cirrhosis. AME Med J 2017;2:39.

