
Role of Wnt inhibitor Apcdd1 in retinal angiogenesis and barrier formation
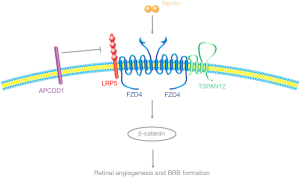
Angiogenesis and blood-retinal barrier (BRB) formation/maintenance are required for normal function of the eye. These processes are controlled by canonical Wnt (β-catenin-dependent) signaling in retinal endothelial cells (ECs) (1). In the retinal vasculature, canonical Wnt signaling is mediated by the ligand NDP (Norrie disease protein) and a receptor complex composed of FZD4 (frizzled class receptor 4), LRP5 (low-density lipoprotein receptor-related protein 5), and the tetraspanin family member TSPAN12 (2-6). NDP, FZD4, LRP5 and TSPAN12 mutant mice had been reported by several groups to exhibit similar vascular defects characterized by a lack of intraretinal blood vessels and BRB defects (2,7). Additionally, in mutant mice where NDP/FZD4 endo-lysosomal trafficking is inhibited specifically in ECs, similar morphological defects are observed in the retinal vasculature (8). These defects in mice resemble those observed in human patients with FEVR (familial exudative vitreoretinopathy) disease. In these mutant mice discussed, canonical Wnt signaling is downregulated, yet, canonical Wnt signaling upregulation has not been well studied.
Recently, a paper published in Neuron by Mazzoni et al. presented a mouse model lacking the canonical Wnt inhibitor Apcdd1 (adenomatosis polyposis coli down-regulated 1) and characterized its role in retinal angiogenesis as well as barrier formation at different stages during early development (Figure 1) (9). In this paper, Mazzoni et al. first examined the expression pattern of Apcdd1 in the retina by RNA in situ hybridization from P6-P17 in mice. Apcdd1 is mainly expressed in retinal ECs but not in pericytes or other neuronal markers, indicating a potential role of Apcdd1 in retinal angiogenesis. Mazzoni et al. comprehensively examined retinal blood vessels from Apcdd1 mutant mice (12). Although there are no differences detected between control and Apcdd1 mutants at P5, knockout mice have significantly increased retinal blood vessel density from P10–P12. This vascular overgrowth phenotype contrasts canonical Wnt signaling loss-of-function phenotype. Additionally, Apcdd1 has been reported to be downregulated in retinas with mutant Norrin or Lrp5 (13). The increased vessel density observed in Apcdd1 mutants may be due to upregulated canonical Wnt signaling. Interestingly, Mazzoni et al. identified upregulated Sox17 (Wnt/β-catenin target gene), increased pLRP6 and more active β-catenin at P10 in Apcdd1 mutant mice (12). These results support Apcdd1 acts as a negative regulator for canonical Wnt signaling in retinal angiogenesis. However, this overgrowth phenotype is only restricted in superficial vascular layer and eventually resolves after P14, suggesting that Apcdd1 mutants have a milder defect than Norrin, FZD4, LRP5, and TSPAN12 mutants. Their findings provide evidence that canonical Wnt signaling needs to be precisely controlled to ensure normal vascular development in the retina.
There are two reasons for retinal vascular overgrowth: retinal EC over proliferation or delayed vessel pruning. Canonical Wnt signaling has been reported to be involved in the both processes (14,15). For example, less EC proliferation and more vessel regression have been reported in Norrin mutant mice (15). However, Mazzoni et al. performed EdU assay on Apcdd1 mutant mice from P8–P12 and did not find any evidence of over proliferation (9). On the other hand, some of newly formed ECs did not go through the pruning process in Apcdd1 mutant retinas, which result hyper-vasculature. These results suggest Apcdd1 negatively controls canonical Wnt signaling but only in the process of pruning.
Canonical Wnt signaling also controls development and maintenance of the BRB (3,5,7). It is possible that BRB formation is affected in Apcdd1 mutant mice. Mazzoni et al. used a low-molecular weight biocytin tracer to test paracellular BRB permeability in Apcdd1 mutant mice (9). These results demonstrated that Apcdd1 mutant mice from P10–P14 formed stronger paracellular BRB. Within EC junctions, the occludin mRNA level was not altered but its protein level increased in Apcdd1 mutants, indicating Apcdd1 affects BRB formation by regulating occludin protein stability. Consistent with the vessel overgrowth phenotype, stronger paracellular BRB permeability also disappeared at later stages. The BRB is also characterized by a low transcellular trafficking rate. Mazzoni et al. tested this trafficking rate in Apcdd1 mutant mice by intravenous injection of albumin, a transcellular tracer. However, there was no significant change for transcellular trafficking in the retinal ECs of Apcdd1 mutant mice. Interestingly, isolated brain ECs showed similar decreased paracellular permeability as in the BRB but increased transcellular permeability, which was inhibited by adding Wnt3a.
Mazzoni et al. also generated a mouse model for Apcdd1 gain-of-function specifically in ECs (9). These endothelial Apcdd1 overexpression mice display opposite phenotypes to Apcdd1 knockout mice, including decreased retinal blood vessel density and higher paracellular BRB permeability. All these data support Apcdd1 playing an important role in regulating retinal angiogenesis and BRB formation.
There are still several questions remaining for the role of Apcdd1. First, although Apcdd1 mutant mice show phenotypes at early stage in retinas, they all revert back to normal at later time points. Is there a parallel pathway compensating for Apcdd1? Are there any phenotypes in ECs from other organs? Second, Apcdd1 has been previously reported to interact with Wnt3a and Lrp5 (10). Does it also interact with Norrin or other molecules in canonical Wnt signaling? Third, it is reported that Apcdd1 mRNA level is decreased in Norrin and Lrp5 mutant retinas (13). Is Apcdd1 a direct target for Norrin/Lrp5 or due to a complicated feedback loop? Forth, does inhibiting Apcdd1 rescue defects from Norrin/FZD4/LRP5/Tspan12 mutant mice? Addressing these questions will not only help further our understanding of how canonical Wnt signaling is regulated but also provide new therapeutic avenues for treating diseases that result from loss of canonical Wnt signaling, such as FEVR disease.
Acknowledgements
The author would like to thank Dr. Maria B. Lai for comments and discussions.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Ziwei Li (Department of Surgery, The First Hospital Affiliated to Kunming Medical School, Kunming, China).
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.02.05). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Daneman R, Agalliu D, Zhou L, et al. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A 2009;106:641-6. [Crossref] [PubMed]
- Junge HJ, Yang S, Burton JB, et al. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell 2009;139:299-311. [Crossref] [PubMed]
- Wang Y, Rattner A, Zhou Y, et al. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 2012;151:1332-44. [Crossref] [PubMed]
- Ye X, Wang Y, Cahill H, et al. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell 2009;139:285-98. [Crossref] [PubMed]
- Zhou Y, Wang Y, Tischfield M, et al. Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest 2014;124:3825-46. [Crossref] [PubMed]
- Xu Q, Wang Y, Dabdoub A, et al. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell 2004;116:883-95. [Crossref] [PubMed]
- Ye X, Wang Y, Nathans J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol Med 2010;16:417-25. [Crossref] [PubMed]
- Zhang C, Lai MB, Khandan L, et al. Norrin-induced Frizzled4 endocytosis and endo-lysosomal trafficking control retinal angiogenesis and barrier function. Nat Commun 2017;8:16050. [Crossref] [PubMed]
- Mazzoni J, Smith JR, Shahriar S, et al. The Wnt Inhibitor Apcdd1 Coordinates Vascular Remodeling and Barrier Maturation of Retinal Blood Vessels. Neuron 2017;96:1055-69.e6. [Crossref] [PubMed]
- Shimomura Y, Agalliu D, Vonica A, et al. APCDD1 is a novel Wnt inhibitor mutated in hereditary hypotrichosis simplex. Nature 2010;464:1043-7. [Crossref] [PubMed]
- Lai MB, Zhang C, Shi J, et al. TSPAN12 Is a Norrin Co-receptor that Amplifies Frizzled4 Ligand Selectivity and Signaling. Cell Rep 2017;19:2809-22. [Crossref] [PubMed]
- Turakainen H, Saarimäki-Vire J, Sinjushina N, et al. Transposition-based method for the rapid generation of gene-targeting vectors to produce Cre/Flp-modifiable conditional knock-out mice. PLoS One 2009;4:e4341 [Crossref] [PubMed]
- Chen J, Stahl A, Krah NM, et al. Retinal expression of Wnt-pathway mediated genes in low-density lipoprotein receptor-related protein 5 (Lrp5) knockout mice. PLoS One 2012;7:e30203 [Crossref] [PubMed]
- Masckauchán TN, Shawber CJ, Funahashi Y, et al. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis 2005;8:43-51. [Crossref] [PubMed]
- Zuercher J, Fritzsche M, Feil S, et al. Norrin stimulates cell proliferation in the superficial retinal vascular plexus and is pivotal for the recruitment of mural cells. Hum Mol Genet 2012;21:2619-30. [Crossref] [PubMed]
Cite this article as: Zhang C. Role of Wnt inhibitor Apcdd1 in retinal angiogenesis and barrier formation. AME Med J 2018;3:34.

