Robotic-assisted right middle lobectomy
Clinical data
Medical history
A ground-glass opacity (GGO) was detected in the medial segment of the right middle lung lobe of a 48-year-old man about one year ago during a regular medical examination. He did not have any symptoms (e.g., chest tightness, shortness of breath, cough, or expectoration) at that time; therefore, we suggested he undergo regular re-examinations. Result of a computed-tomography (CT) scan one month ago still indicated a 0.9-cm GGO in the same position. The patient still had no symptoms. Positron emission tomography-CT showed hypermetabolism in this nodule. The patient’s physical performance was good, and his appetite, sleep, urination, and defecation were normal. His body weight did not change significantly. He denied smoking or alcohol abuse, and family history.
Physical examination
A complete physical examination was performed when the patient was admitted. No abnormity was found. No lymph nodes were palpable in the neck, axilla, or below the clavicle.
Auxiliary examination
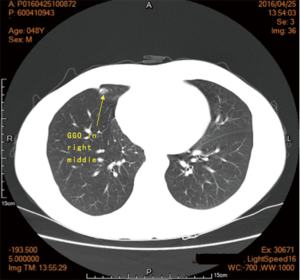
Chest CT showed a 1.1-cm elliptic GGO in the right middle lung lobe, with clear margins, without lobulation sign, spiculation or vacuole sign (Figure 1). No abnormity was found in the lung hila. No abnormal lymph nodes were found in the mediastinum.
PET/CT showed hypermetabolism in the nodule of the right middle lung lobe.
Abdominal ultrasound scan, bone scan, cranial magnetic resonance imaging, echocardiogram, and pulmonary function were normal. In addition, blood routine test, hepatorenal function, and blood gases were normal.
Pre-operative preparation
The results of imaging, including PET/CT, suggested that the GGO was considered to be malignant. The lesion was in medial segment of the right middle lung lobe, near the hilum; therefore, segmentectomy and wedge resection were not suitable. After the preoperative discussion and agreement of the patient, we decided to perform robotic-assisted right middle lobectomy.
The patient was a middle-aged man in generally good condition, without chronic disease, smoking or alcohol abuse, so the preoperative preparation was quite simple. During preoperative education, we described his condition and the surgical method, as well as situations that may occur after surgery. We told him to practice elimination while in bed. We taught him pulmonary function training and how to cough and expectorate after surgery. Eating and drinking are routinely forbidden after 9 pm the day before the operation.
Surgical procedures
Anesthesia and body position
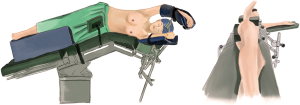
The patient was first placed in the supine position. After combined intravenous and inhalation anesthesia, the patient was placed in a right lateral decubitus position under double-lumen endotracheal intubation, with his hands in front of his head. Then he was placed in the jackknife position and provided with single-lung (left) ventilation. After the patient was fixed tightly, the operation table was turned about 20° towards the patient’s back (Figure 2).
Incisions
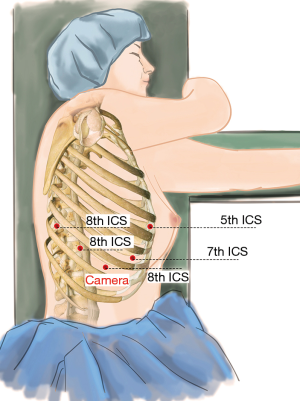
A 12-mm camera trocar was placed in the 8th intercostal space (ICS) at the right mid axillary line, three 8-mm working trocars were placed separately in the 5th ICS at the right anterior axillary line (#1 arm), 8th ICS at the right posterior axillary line (#2 arm), and the right 8th ICS (#3 arm), 2 cm from the spine. A 12-mm auxiliary incision was made in the 7th ICS at the right posterior axillary line (Figure 3). Then we created 8–10 mmHg artificial pneumothorax using CO2. The patient-side cart was connected over the patient’s head. The #1 arm (right hand) was connected to permanent cautery hook, and the #2 arm (left hand) was connected to fenestrated bipolar forceps.
Procedures
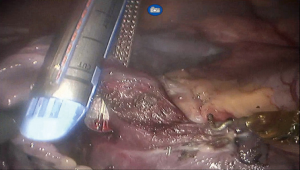
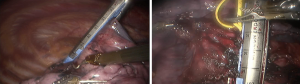
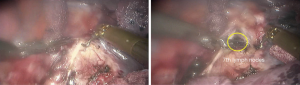
See Figures 4,5,6,7,8,9,10,11,12.
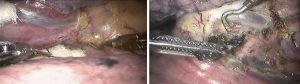
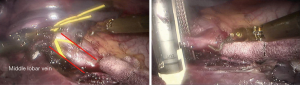
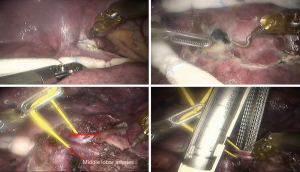
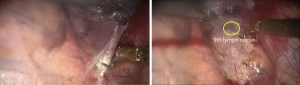
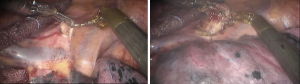
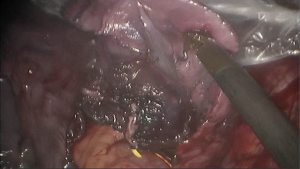
Finally, the thoracic cavity was washed with warm water, and the right lung was ventilated. No air leakage or bleeding was observed. We placed an indwelling 28# thoracic drainage tube and a thoracic micro-tube at 8th ICS and 9th ICS, respectively. Then all incisions were closed.
Postoperative treatment
Postoperative treatment is similar to that given after video-assisted lobectomy. No complication was observed. The patient ate and took part in out-of-bed activities on the first day after surgery, and was discharged on the fourth day after surgery, with the thoracic drainage tube withdrawn. The thoracic micro-tube was withdrawn on 14th day after surgery.
Pathologic diagnosis was lung adenocarcinoma in situ (1 cm × 0.5 cm × 0.5 cm) with focal micro-invasion. No cancer cells were detected at the bronchial stump or lymph nodes.
Discussion
Because of the aplasia of fissures and the adhesion of lymph nodes around the vessels and bronchus, right middle lobectomy can be difficult (1). The stability and dexterity of the da Vinci Surgical System make it suitable for this procedure (2). Especially in systematic lymphadenectomy (3), the high-resolution three dimensional view makes it easier to completely remove lymph nodes thoroughly with less injury to nearby tissues.
Appropriate body position and incisions are key points of this surgery. We choose the lateral decubitus position and elevated the chest to make the surgical field clearer (4). After several attempts, we choose these incision locations that make it possible for the camera and robotic arms to cover the entire thoracic cavity. The auxiliary incision should be chosen to be convenient for placing instruments, such as the Endo GIATM and the specimen bag, and to minimize mutual interference between the robotic arms.
Regarding the choice of instruments, we use three arms (5), including a camera lens, a permanent cautery hook and a fenestrated bipolar forceps to meet the need for cutting, dissociation, coagulation, and pickup.
The case was typical, dealing with veins, arteries, bronchus, and fissures successively. However, we should pay attention to potential anatomic variations of these structures. In addition, the assistant should cooperate well with the surgeon and be experienced in thoracoscopic surgery and thoracotomy. In emergency circumstances, such as rupture of the main vessels, the assistant should be able to handle the situation independently under thoracoscopy or even open the chest immediately.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.01.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gaudet MA, D'Amico TA. Thoracoscopic Lobectomy for Non-small Cell Lung Cancer. Surg Oncol Clin N Am 2016;25:503-13. [Crossref] [PubMed]
- Wei B, Eldaif SM, Cerfolio RJ. Robotic Lung Resection for Non-Small Cell Lung Cancer. Surg Oncol Clin N Am 2016;25:515-31. [Crossref] [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-8; discussion 208-9. [Crossref] [PubMed]
- Yan TD. Surgical atlas of thoracoscopic lobectomy and segmentectomy. Ann Cardiothorac Surg 2014;3:183-91. [PubMed]
- Park BJ, Yang HX, Woo KM, et al. Minimally invasive (robotic assisted thoracic surgery and video-assisted thoracic surgery) lobectomy for the treatment of locally advanced non-small cell lung cancer. J Thorac Dis 2016;8:S406-13. [Crossref] [PubMed]
Cite this article as: Chen X, Yang S, Guo W, Jin R, Zhang Y, Wu H, Du H, Han D, Chen K, Xiang J, Li H. Robotic-assisted right middle lobectomy. AME Med J 2017;2:8.