
Proton pump inhibitors in liver cirrhosis: a review of benefits and harms
Introduction
Liver cirrhosis
Liver cirrhosis is the end-stage of liver disease, in which the liver is gradually shrunk and is separated by broad fibrotic bands related to extensive necrosis and regenerative nodules of liver cells (1). Liver cirrhosis can result in hepatic encephalopathy (HE) and spontaneous bacterial peritonitis (SBP), which are associated with a high mortality (2). The overgrowth of intestinal bacteria and the development of bacterial translocation increase the risk of infection and HE in cirrhotic patients (3-5). The severity of liver cirrhosis is positively associated with the development of small intestinal bacterial overgrowth (6). On the other hand, intestinal permeability is often increased in patients with cirrhosis, which leads to the translocation of bacteria and endotoxins into the portal venous system, thereby impairing the immunity (7,8).
Proton pump inhibitors (PPIs)
PPIs are effective acid suppressants that are widely prescribed for managing various acid related disorders. As the front-line choice of therapy, they block gastric acid secretion through inhibiting the H+/K+ ATPase of parietal cells (9) and are metabolized in the liver by the CYP450 cytochrome (10).
The use of PPIs is common in cirrhotic patients at present. However, the prescription of PPIs in such patients is often lacking of specific indications, such as gastroesophageal reflux disease, peptic ulcers, non-variceal upper gastrointestinal bleeding, and bleeding prophylaxis in selected users of nonsteroidal anti-inflammatory drugs (11-15). Recent studies suggested that the use of PPIs might decrease the abundance and diversity of gut microbiota and lead to the growth of pathogens and the overgrowth of unhealthy species (16-18). Long-term use of PPIs may increase the incidence of bone fracture (19), clostridium difficile infection (CDI) (20,21), HE (22), and SBP (23,24), which may be related to the overgrowth of bacteria (3,25).
The purpose of this paper is to review the potential benefits and harms of PPIs in liver cirrhosis.
Potential benefits of PPIs
Peptic ulcer treatment and helicobacter pylori eradication
Recent studies found that liver cirrhosis increased the risk of peptic ulcers (26,27). The prevalence of peptic ulcers is higher in decompensated cirrhosis than in compensated cirrhosis (28). Currently, PPIs are the mainstay treatment option of peptic ulcers in the general population (29-31).
Cirrhotic patients also have a high proportion of helicobacter pylori infection. Helicobacter pylori promotes the conversion of urea into ammonia, which enters into the systemic circulation. Helicobacter pylori infection contributes to the development of hyperammonemia (32,33) and subsequent episodes of HE (34,35) in cirrhosis. Helicobacter pylori eradication has been improved by PPIs-based triple therapy (36). Notably, blood ammonia concentration is significantly reduced after PPIs-based triple treatment in cirrhotic patients (34,35). As well known, the application of PPIs triple therapy can effectively treat helicobacter pylori infection which can increase the risk of HE. Therefore, it might be true that PPIs reduce the risk of HE in patients with helicobacter pylori infection (Figure 1).
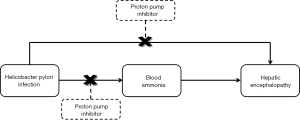
A meta-analysis by Vergara et al. pointed out that helicobacter pylori infection was a risk factor for developing peptic ulcers in cirrhotic patients (37). Similarly, Calvet et al. showed that helicobacter pylori seropositivity was an independent risk factor for increasing the rate of peptic ulcers in patients with cirrhosis [odds ratio (OR)=1.7; 95% confidence interval (CI) =1.02–2.81] (38). Thus, helicobacter pylori eradication with PPIs treatment should be necessary. By contrast, some studies showed that the prevalence of helicobacter pylori infection was not significantly different between cirrhotic patients with and without peptic ulcers (39,40). Additionally, a recent prospective cohort study found that helicobacter pylori eradication could not effectively prevent from the recurrence of peptic ulcer in patients with cirrhosis (41).
Endoscopic treatment
Endoscopic treatment, including endoscopic variceal ligation (EVL), endoscopic injection sclerotherapy (EIS), and endoscopic variceal obturation (EVO), can effectively prevent and control variceal haemorrhage in patients with liver cirrhosis. According to the current U.K. practice guideline, due to the potential harms of PPIs, they are not recommended for acute variceal bleeding unless patients are accompanied by peptic ulcer (42). However, post-EVL or EIS ulcer bleeding is life-threatening and gastric acid is a significant risk factor which delays post-EIS ulceration healing (43). Therefore, PPIs may be appropriate after endoscopic treatment on the basis of the following considerations.
First, some evidence regarding the use of PPIs after EIS was controversial. Some studies found that omeprazole was effective for preventing from and healing of post-EIS ulcerations (44-46). In contrast, Garg et al. revealed that the use of omeprazole was not significantly effective in preventing from post-EIS ulceration in patients with portal hypertension (47).
Second, the evidence uniformly supported the use of PPIs for reducing the risk of complications after EVL. A randomized controlled trial demonstrated that pantoprazole reduced the size of post-EVL ulcerations, rather than the number of ulcerations or symptoms (48). Hidaka et al. suggested that the long-term use of PPIs decreased the risk of variceal hemorrhage or severe medical complications after EVL (49). A more recent study with 505 cirrhotic patients also showed that non-use of PPIs was an independent risk factor associated with bleeding after prophylactic EVL by multivariate logistic analysis (50).
Third, to our knowledge, the evidence from the only one study demonstrated that PPIs treatment was effective for prolonging the re-bleeding interval (P=0.008), but not the re-bleeding rate after EVO in cirrhotic patients (51).
In summary, PPIs may be appropriate for reducing the risk of complications after endoscopic treatment.
Potential harms of PPIs
Osteoporosis and bone fracture
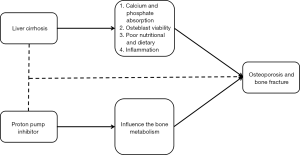
Osteoporosis refers to an abnormal condition that the bone becomes weak and can be easily broken, reduces the life quality, and increases the risk of bone fracture. Some researchers suggested that the use of PPIs should increase the risk of bone fracture (19,52) and observed a dose- or duration-response effect of PPIs on the risk of bone fracture (53-57). The potential mechanism should be that PPIs influenced the bone metabolism by inhibiting the vacuolar H+-ATPase of osteoclasts, thereby leading to the occurrence of osteoporosis (58). By contrast, others suggested a reduced relationship between PPIs use and bone fracture after adjusting the confounding factors (59,60). Three meta-analyses showed that the use of PPIs was a risk factor of bone fracture, but the occurrence of bone fracture might be unrelated to the dosage or duration of PPIs (61-63). In addition, Itoh et al. indicated that bisphosphonate combined with PPIs increased the bone mineral density than bisphosphonate alone in patients with osteoporosis (64).
Liver cirrhosis is a risk factor for the development of osteoporosis and bone fracture. In a large nationwide population-based and case-control study, Tsai et al. found that cirrhotic patients had a higher risk of bone fracture than non-cirrhotic patients (65). Similarly, Bang et al. also found that liver cirrhosis was a predisposing factor of bone fracture (66). The potential mechanisms include (I) a reduced calcium and phosphate absorption (67); (II) effect of bilirubin on the osteoblast viability (68); (III) poor nutritional and dietary (69); and (IV) effect of increased inflammation on the bone mineral density (70,71). By contrast, there was no significant association between bone mineral density and primary biliary cirrhosis (72,73).
Taken together, the current evidence indicated two important aspects: (I) an association between PPIs and bone fracture; and (II) an association between liver cirrhosis and bone fracture. Thus, we hypothesized that the use of PPIs might aggravate the risk of bone fracture in cirrhosis (Figure 2), but the direct evidence was lacking.
CDI
The incidence of CDI is being increased in this world. In the general population, the association between PPIs with CDI is a bit controversial. Some studies showed that PPIs therapy was prone to CDI (74-77) due to the overgrowth of gut microbiota (17). PPIs therapy elevates the pH value and promotes the survival of clostridia species (78). By contrast, a population-based study showed that PPIs were not associated with CDI after adjusting the confounding factors (79).
Liver cirrhosis leads to a higher risk of healthcare-associated and hospital-acquired bacterial infections (80). The incidence of CDI has been increased among the cirrhotic patients. Cirrhotic patients with CDI are prone to worse outcomes (20,81-83). A large study, using Nationwide Inpatient Sample database, revealed that CDI was a predictive factor of mortality in cirrhotic patients (20). CDI was prone to higher mean length of stay and hospital charges in cirrhotic patients (20). A retrospective study with 162 cirrhotic patients admitted to a tertiary care center revealed that the proportion of PPIs use was significantly higher in cirrhotic patients with CDI than in those without CDI (20). Besides, a multivariable analysis demonstrated that PPIs use promoted the development of CDI in cirrhotic outpatients (20). Certainly, further studies are needed to analyze a causal relationship between PPIs and CDI in patients with cirrhosis.
SBP
As one of the serious complications of liver cirrhosis, SBP is related to a high morbidity and mortality in the absence of appropriate therapy. PPIs can promote the small intestinal bacterial overgrowth (84) and altered intestinal motility, which might be the pathogenesis of SBP. Some evidence had shown that PPIs treatment was a predisposing factor for the development of SBP in patients with cirrhosis (12,24,85-88). Two case-control studies found that cirrhotic patients with SBP had a higher proportion of PPIs use than those without SBP (85,89). Three meta-analyses identified that the use of PPIs increased the incidence of SBP in cirrhotic patients (24,86,90). Xu et al. (24) showed that PPIs increased the risk of SBP. Similar results were obtained by Deshpande et al. that the application of acid-suppressive drugs increased the occurrence of SBP in cirrhotic patients (86). Besides, the prescription of PPIs had a higher risk of SBP compared with histamine H2 receptor blockade therapy (86). The occurrence of SBP might be related to the dosage and duration of PPIs. The Taiwan National Health Insurance Research database showed a significant association between long-term PPIs treatment (>180 days) and incidence of SBP [adjusted hazard ratio (aHR) =2.28, 95% CI =1.37–3.78] by using multivariate Cox regression (87). Similar results were shown by Chang et al. that prolonged PPIs use increased the incidence of SBP in cirrhotic patients (88).
In contrast, more recently, in a multicenter prospective study from Argentina, Terg et al. found that the prescription of PPIs was not significantly different between patients with and without SBP (91). A larger meta-analysis with 8,145 patients confirmed that PPIs use was prone to a higher risk of SBP only in case-control studies rather than in cohort studies (92). The association between SBP and PPIs would be diminished, if only high-quality data were analyzed (93).
HE
HE is a complex, reversible, and lethal syndrome of neuropsychiatric abnormalities due to acute and chronic liver dysfunction or a variety of portosystemic shunt. The impact of PPIs on the development of HE may be explained by the two following mechanisms.
First, high blood ammonia is one of the potential pathogeneses of HE (94). High ammonia levels interfere with the brain energy metabolism and produce the central inhibitory effect by an imbalance of excitability and inhibitory neurotransmitter. Additionally, small intestinal bacterial overgrowth is a risk factor of minimal HE in cirrhotic patients (95). PPIs alter the gastrointestinal motility and affect the mucosal barrier (96), thereby increasing the absorption of nitrogenous substances. PPIs also promote the overgrowth and translocation of bacteria (84). Thus, PPIs may increase the risk of HE.
Second, PPIs therapy decreases the absorption of vitamin B12 and magnesium (97,98), thereby impairing the cognitive ability (99). Some evidence found that the use of PPIs was associated with a high risk of dementia and Alzheimer’s disease (100,101). Thus, PPIs may aggravate the risk of HE in cirrhotic patients (Figure 3).
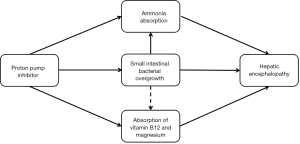
Recently, some studies uniformly proved that the use of PPIs contributed to a higher risk of HE in liver cirrhosis. A small retrospective case-control study showed that the use of PPIs was an independent risk factor for HE occurrence (102). In a case-control study, using the Taiwan National Health Insurance beneficiaries database, Tsai et al. also found that the use of PPIs in patients with cirrhosis was prone to the occurrence of HE (22). Moreover, there was a dose-dependent relationship between PPIs and HE (22). Another retrospective study involving 865 cirrhotic patients with ascites demonstrated that PPIs therapy was a risk factor for the development of HE (aHR =1.36, 95% CI =1.01–1.84), particularly the occurrence of overt HE (aHR =1.88, 95% CI =1.21–1.91) (103). The cumulative risk of first-time HE is higher in patients with PPIs therapy than in those without PPIs therapy (31% vs. 25%) (103).
Conclusions
The widespread prescription of PPIs in patients with cirrhosis needs to be concerned. On the one hand, PPIs can effectively control the occurrence of complications after endoscopic treatment and reduce the risk of ulcers related to the helicobacter pylori infection in cirrhotic patients, which are the potential benefits. On the other hand, the use of PPIs may increase the incidence of serious complications, such as bone fracture, CDI, SBP, and HE, which are the potential harms. However, the potential mechanisms regarding how PPIs increased the mortality remained uncertain. In future, more researches are needed to understand the impact of PPIs in liver cirrhosis.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.03.04). Andrea Mancuso serves as an unpaid editorial board member of AME Medical Journal. Xingshun Qi serves as an Editor-in-Chief of AME Medical Journal. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hori T, Ogura Y, Onishi Y, et al. Systemic hemodynamics in advanced cirrhosis: Concerns during perioperative period of liver transplantation. World J Hepatol 2016;8:1047-60. [Crossref] [PubMed]
- D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217-31. [Crossref] [PubMed]
- Bauer TM, Steinbrückner B, Brinkmann FE, et al. Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol 2001;96:2962-7. [Crossref] [PubMed]
- Yang CY, Chang CS, Chen GH. Small-intestinal bacterial overgrowth in patients with liver cirrhosis, diagnosed with glucose H2 or CH4 breath tests. Scand J Gastroenterol 1998;33:867-71. [Crossref] [PubMed]
- Zhang Y, Feng Y, Cao B, et al. The effect of small intestinal bacterial overgrowth on minimal hepatic encephalopathy in patients with cirrhosis. Arch Med Sci 2016;12:592-6. [Crossref] [PubMed]
- Pande C, Kumar A, Sarin SK. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment Pharmacol Ther 2009;29:1273-81. [Crossref] [PubMed]
- Cariello R, Federico A, Sapone A, et al. Intestinal permeability in patients with chronic liver diseases: Its relationship with the aetiology and the entity of liver damage. Dig Liver Dis 2010;42:200-4. [Crossref] [PubMed]
- Aguirre Valadez JM, Rivera-Espinosa L, Méndez-Guerrero O, et al. Intestinal permeability in a patient with liver cirrhosis. Ther Clin Risk Manag 2016;12:1729-48. [Crossref] [PubMed]
- Sachs G, Shin JM, Briving C, et al. The pharmacology of the gastric acid pump: the H+,K+ ATPase. Annu Rev Pharmacol Toxicol 1995;35:277-305. [Crossref] [PubMed]
- Furuta T, Shirai N, Sugimoto M, et al. Pharmacogenomics of proton pump inhibitors. Pharmacogenomics 2004;5:181-202. [Crossref] [PubMed]
- Jairath V, Rehal S, Logan R, et al. Acute variceal haemorrhage in the United Kingdom: patient characteristics, management and outcomes in a nationwide audit. Dig Liver Dis 2014;46:419-26. [Crossref] [PubMed]
- Min YW, Lim KS, Min BH, et al. Proton pump inhibitor use significantly increases the risk of spontaneous bacterial peritonitis in 1965 patients with cirrhosis and ascites: a propensity score matched cohort study. Aliment Pharmacol Ther 2014;40:695-704. [Crossref] [PubMed]
- Chavez-Tapia NC, Tellez-Avila FI, Garcia-Leiva J, et al. Use and overuse of proton pump inhibitors in cirrhotic patients. Med Sci Monit 2008;14:CR468-72. [PubMed]
- Mandorfer M, Bota S, Schwabl P, et al. Proton pump inhibitor intake neither predisposes to spontaneous bacterial peritonitis or other infections nor increases mortality in patients with cirrhosis and ascites. PLoS One 2014;9:e110503. [Crossref] [PubMed]
- Barrison AF, Jarboe LA, Weinberg BM, et al. Patterns of proton pump inhibitor use in clinical practice. Am J Med 2001;111:469-73. [Crossref] [PubMed]
- Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016;65:749-56. [Crossref] [PubMed]
- Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65:740-8. [Crossref] [PubMed]
- Bajaj JS, Ratliff SM, Heuman DM, et al. Proton pump inhibitors are associated with a high rate of serious infections in veterans with decompensated cirrhosis. Aliment Pharmacol Ther 2012;36:866-74. [PubMed]
- van der Hoorn MM, Tett SE, de Vries OJ, et al. The effect of dose and type of proton pump inhibitor use on risk of fractures and osteoporosis treatment in older Australian women: A prospective cohort study. Bone 2015;81:675-82. [Crossref] [PubMed]
- Bajaj JS, Ananthakrishnan AN, Hafeezullah M, et al. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: A national and tertiary center perspective. Am J Gastroenterol 2010;105:106-13. [Crossref] [PubMed]
- Trifan A, Stoica O, Stanciu C, et al. Clostridium difficile infection in patients with liver disease: a review. Eur J Clin Microbiol Infect Dis 2015;34:2313-24. [Crossref] [PubMed]
- Tsai CF, Chen MH, Wang YP, et al. Proton Pump Inhibitors Increase Risk for Hepatic Encephalopathy in Patients With Cirrhosis in A Population Study. Gastroenterology 2017;152:134-41. [Crossref] [PubMed]
- Kwon JH, Koh SJ, Kim W, et al. Mortality associated with proton pump inhibitors in cirrhotic patients with spontaneous bacterial peritonitis. J Gastroenterol Hepatol 2014;29:775-81. [Crossref] [PubMed]
- Xu HB, Wang HD, Li CH, et al. Proton pump inhibitor use and risk of spontaneous bacterial peritonitis in cirrhotic patients: a systematic review and meta-analysis. Genet Mol Res 2015;14:7490-501. [Crossref] [PubMed]
- Bajaj JS, Cox IJ, Betrapally NS, et al. Systems biology analysis of omeprazole therapy in cirrhosis demonstrates significant shifts in gut microbiota composition and function. Am J Physiol Gastrointest Liver Physiol 2014;307:G951-7. [Crossref] [PubMed]
- Chen LS, Lin HC, Hwang SJ, et al. Prevalence of gastric ulcer in cirrhotic patients and its relation to portal hypertension. J Gastroenterol Hepatol 1996;11:59-64. [Crossref] [PubMed]
- Chen LS, Lin HC, Lee FY, et al. Prevalence of duodenal ulcer in cirrhotic patients and its relation to Helicobacter pylori and portal hypertension. Zhonghua Yi Xue Za Zhi (Taipei) 1995;56:226-31. [PubMed]
- Wu CS, Lin CY, Liaw YF. Helicobacter pylori in cirrhotic patients with peptic ulcer disease: a prospective, case controlled study. Gastrointest Endosc 1995;42:424-7. [Crossref] [PubMed]
- Tytgat GN. Etiopathogenetic principles and peptic ulcer disease classification. Dig Dis 2011;29:454-8. [Crossref] [PubMed]
- Kim HU. Diagnostic and Treatment Approaches for Refractory Peptic Ulcers. Clin Endosc 2015;48:285-90. [Crossref] [PubMed]
- Tang RS, Wu JC. Managing peptic ulcer and gastroesophageal reflux disease in elderly Chinese patients--focus on esomeprazole. Clin Interv Aging 2013;8:1433-43. [PubMed]
- Miyaji H, Ito S, Azuma T, et al. Effects of Helicobacter pylori eradication therapy on hyperammonaemia in patients with liver cirrhosis. Gut 1997;40:726-30. [Crossref] [PubMed]
- Plevris JN, Morgenstern R, Hayes PC, et al. Hyperammonaemia in cirrhosis and Helicobacter pylori infection. Lancet 1995;346:1104. [Crossref] [PubMed]
- Chen SJ, Wang LJ, Zhu Q, et al. Effect of H pylori infection and its eradication on hyperammo-nemia and hepatic encephalopathy in cirrhotic patients. World J Gastroenterol 2008;14:1914-8. [Crossref] [PubMed]
- Agrawal A, Gupta A, Chandra M, et al. Role of Helicobacter pylori infection in the pathogenesis of minimal hepatic encephalopathy and effect of its eradication. Indian J Gastroenterol 2011;30:29-32. [Crossref] [PubMed]
- Jung SW, Lee SW, Hyun JJ, et al. Efficacy of Helicobacter pylori eradication therapy in chronic liver disease. Dig Liver Dis 2009;41:134-40. [Crossref] [PubMed]
- Vergara M, Calvet X, Roqué M. Helicobacter pylori is a risk factor for peptic ulcer disease in cirrhotic patients. A meta-analysis. Eur J Gastroenterol Hepatol 2002;14:717-22. [Crossref] [PubMed]
- Calvet X, Navarro M, Gil M, et al. Epidemiology of peptic ulcer disease in cirrhotic patients: role of Helicobacter pylori infection. Am J Gastroenterol 1998;93:2501-7. [Crossref] [PubMed]
- Tsai CJ. Helicobacter pylori infection and peptic ulcer disease in cirrhosis. Dig Dis Sci 1998;43:1219-25. [Crossref] [PubMed]
- Zullo A, Rinaldi V, Meddi P, et al. Helicobacter pylori infection in dyspeptic cirrhotic patients. Hepatogastroenterology 1999;46:395-400. [PubMed]
- Tzathas C, Triantafyllou K, Mallas E, et al. Effect of Helicobacter pylori eradication and antisecretory maintenance therapy on peptic ulcer recurrence in cirrhotic patients: a prospective, cohort 2-year follow-up study. J Clin Gastroenterol 2008;42:744-9. [Crossref] [PubMed]
- Tripathi D, Stanley AJ, Hayes PC, et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015;64:1680-704. [Crossref] [PubMed]
- Johlin FC, Labrecque DR, Neil GA. Omeprazole heals mucosal ulcers associated with endoscopic injection sclerotherapy. Dig Dis Sci 1992;37:1373-6. [Crossref] [PubMed]
- Shephard H, Barkin JS. Omeprazole heals mucosal ulcers associated with endoscopic injection sclerotherapy. Gastrointest Endosc 1993;39:474-5. [Crossref] [PubMed]
- Jaspersen D, Körner T, Schorr W, et al. Omeprazole in the management of sclerotherapy-induced esophageal ulcers resistant to H2 blocker treatment. J Gastroenterol 1995;30:128-30. [Crossref] [PubMed]
- Gimson A, Polson R, Westaby D, et al. Omeprazole in the management of intractable esophageal ulceration following injection sclerotherapy. Gastroenterology 1990;99:1829-31. [Crossref] [PubMed]
- Garg PK, Sidhu SS, Bhargava DK. Role of omeprazole in prevention and treatment of postendoscopic variceal sclerotherapy esophageal complications. Double-blind randomized study. Dig Dis Sci 1995;40:1569-74. [Crossref] [PubMed]
- Shaheen NJ, Stuart E, Schmitz SM, et al. Pantoprazole reduces the size of postbanding ulcers after variceal band ligation: a randomized, controlled trial. Hepatology 2005;41:588-94. [Crossref] [PubMed]
- Hidaka H, Nakazawa T, Wang G, et al. Long-term administration of PPI reduces treatment failures after esophageal variceal band ligation: a randomized, controlled trial. J Gastroenterol 2012;47:118-26. [Crossref] [PubMed]
- Kang SH, Yim HJ, Kim SY, et al. Proton Pump Inhibitor Therapy Is Associated With Reduction of Early Bleeding Risk After Prophylactic Endoscopic Variceal Band Ligation: A Retrospective Cohort Study. Medicine (Baltimore) 2016;95:e2903. [Crossref] [PubMed]
- Jang WS, Shin HP, Lee JI, et al. Proton pump inhibitor administration delays rebleeding after endoscopic gastric variceal obturation. World J Gastroenterol 2014;20:17127-31. [Crossref] [PubMed]
- Fraser LA, Leslie WD, Targownik LE, et al. The effect of proton pump inhibitors on fracture risk: report from the Canadian Multicenter Osteoporosis Study. Osteoporos Int 2013;24:1161-8. [Crossref] [PubMed]
- Adams AL, Black MH, Zhang JL, et al. Proton-pump inhibitor use and hip fractures in men: a population-based case-control study. Ann Epidemiol 2014;24:286-90. [Crossref] [PubMed]
- Pouwels S, Lalmohamed A, Souverein P, et al. Use of proton pump inhibitors and risk of hip/femur fracture: a population-based case-control study. Osteoporos Int 2011;22:903-10. [Crossref] [PubMed]
- Abrahamsen B, Eiken P, Eastell R. Proton pump inhibitor use and the antifracture efficacy of alendronate. Arch Intern Med 2011;171:998-1004. [Crossref] [PubMed]
- Chiu HF, Huang YW, Chang CC, et al. Use of proton pump inhibitors increased the risk of hip fracture: a population-based case-control study. Pharmacoepidemiol Drug Saf 2010;19:1131-6. [Crossref] [PubMed]
- Corley DA, Kubo A, Zhao W, et al. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology 2010;139:93-101. [Crossref] [PubMed]
- Jo Y, Park E, Ahn SB, et al. A Proton Pump Inhibitor's Effect on Bone Metabolism Mediated by Osteoclast Action in Old Age: A Prospective Randomized Study. Gut Liver 2015;9:607-14. [Crossref] [PubMed]
- Cea Soriano L, Ruigómez A, Johansson S, et al. Study of the association between hip fracture and acid-suppressive drug use in a UK primary care setting. Pharmacotherapy 2014;34:570-81. [Crossref] [PubMed]
- Reyes C, Formiga F, Coderch M, et al. Use of proton pump inhibitors and risk of fragility hip fracture in a Mediterranean region. Bone 2013;52:557-61. [Crossref] [PubMed]
- Zhou B, Huang Y, Li H, et al. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int 2016;27:339-47. [Crossref] [PubMed]
- Yu EW, Bauer SR, Bain PA, et al. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med 2011;124:519-26. [Crossref] [PubMed]
- Ngamruengphong S, Leontiadis GI, Radhi S, et al. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol 2011;106:1209-18; quiz 1219. [Crossref] [PubMed]
- Itoh S, Sekino Y, Shinomiya K, et al. The effects of risedronate administered in combination with a proton pump inhibitor for the treatment of osteoporosis. J Bone Miner Metab 2013;31:206-11. [Crossref] [PubMed]
- Tsai CF, Liu CJ, Chen TJ, et al. Increased incidence of orthopedic fractures in cirrhotic patients: a nationwide population-based study. J Hepatol 2013;58:706-14. [Crossref] [PubMed]
- Bang UC, Benfield T, Bendtsen F, et al. The risk of fractures among patients with cirrhosis or chronic pancreatitis. Clin Gastroenterol Hepatol 2014;12:320-6. [Crossref] [PubMed]
- Farrington K, Epstein O, Varghese Z, et al. Effect of oral 1,25 dihydroxycholecalciferol on calcium and phosphate malabsorption in primary biliary cirrhosis. Gut 1979;20:616-9. [Crossref] [PubMed]
- Ruiz-Gaspà S, Martinez-Ferrer A, Guañabens N, et al. Effects of bilirubin and sera from jaundiced patients on osteoblasts: contribution to the development of osteoporosis in liver diseases. Hepatology 2011;54:2104-13. [Crossref] [PubMed]
- Cijevschi C, Mihai C, Drug VL, et al. Osteoporosis in liver cirrhosis--overview. Rev Med Chir Soc Med Nat Iasi 2005;109:700-4. [PubMed]
- Gallego-Rojo FJ, Gonzalez-Calvin JL, Muñoz-Torres M, et al. Bone mineral density, serum insulin-like growth factor I, and bone turnover markers in viral cirrhosis. Hepatology 1998;28:695-9. [Crossref] [PubMed]
- Ferrari-Lacraz S, Ferrari S. Do RANKL inhibitors (denosumab) affect inflammation and immunity? Osteoporos Int 2011;22:435-46. [Crossref] [PubMed]
- Ormarsdóttir S, Ljunggren O, Mallmin H, et al. Longitudinal bone loss in postmenopausal women with primary biliary cirrhosis and well-preserved liver function. J Intern Med 2002;252:537-41. [Crossref] [PubMed]
- Newton J, Francis R, Prince M, et al. Osteoporosis in primary biliary cirrhosis revisited. Gut 2001;49:282-7. [Crossref] [PubMed]
- Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. CMAJ 2004;171:33-8. [Crossref] [PubMed]
- Dial S, Delaney JA, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 2005;294:2989-95. [Crossref] [PubMed]
- Janarthanan S, Ditah I, Adler DG, et al. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol 2012;107:1001-10. [Crossref] [PubMed]
- Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007;102:2047-56; quiz 2057. [Crossref] [PubMed]
- Jump RL, Pultz MJ, Donskey CJ. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob Agents Chemother 2007;51:2883-7. [Crossref] [PubMed]
- Lowe DO, Mamdani MM, Kopp A, et al. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin Infect Dis 2006;43:1272-6. [Crossref] [PubMed]
- Sargenti K, Prytz H, Strand A, et al. Healthcare-associated and nosocomial bacterial infections in cirrhosis: predictors and impact on outcome. Liver Int 2015;35:391-400. [Crossref] [PubMed]
- Musa S, Moran C, Rahman T. Clostridium difficile infection and liver disease. J Gastrointestin Liver Dis 2010;19:303-10. [PubMed]
- Singal AK, Salameh H, Kamath PS. Prevalence and in-hospital mortality trends of infections among patients with cirrhosis: a nationwide study of hospitalised patients in the United States. Aliment Pharmacol Ther 2014;40:105-12. [Crossref] [PubMed]
- Garcia-Tsao G, Surawicz CM. Editorial: Clostridium difficile infection: Yet another predictor of poor outcome in cirrhosis. Am J Gastroenterol 2010;105:114-6. [Crossref] [PubMed]
- Lombardo L, Foti M, Ruggia O, et al. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol 2010;8:504-8. [Crossref] [PubMed]
- de Vos M, De Vroey B, Garcia BG, et al. Role of proton pump inhibitors in the occurrence and the prognosis of spontaneous bacterial peritonitis in cirrhotic patients with ascites. Liver Int 2013;33:1316-23. [Crossref] [PubMed]
- Deshpande A, Pasupuleti V, Thota P, et al. Acid-suppressive therapy is associated with spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. J Gastroenterol Hepatol 2013;28:235-42. [Crossref] [PubMed]
- Huang KW, Kuan YC, Luo JC, et al. Impact of long-term gastric acid suppression on spontaneous bacterial peritonitis in patients with advanced decompensated liver cirrhosis. Eur J Intern Med 2016;32:91-5. [Crossref] [PubMed]
- Chang SS, Lai CC, Lee MT, et al. Risk of spontaneous bacterial peritonitis associated with gastric Acid suppression. Medicine (Baltimore) 2015;94:e944. [Crossref] [PubMed]
- Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol 2009;104:1130-4. [Crossref] [PubMed]
- Trikudanathan G, Israel J, Cappa J, et al. Association between proton pump inhibitors and spontaneous bacterial peritonitis in cirrhotic patients - a systematic review and meta-analysis. Int J Clin Pract 2011;65:674-8. [Crossref] [PubMed]
- Terg R, Casciato P, Garbe C, et al. Proton pump inhibitor therapy does not increase the incidence of spontaneous bacterial peritonitis in cirrhosis: a multicenter prospective study. J Hepatol 2015;62:1056-60. [Crossref] [PubMed]
- Yu T, Tang Y, Jiang L, et al. Proton pump inhibitor therapy and its association with spontaneous bacterial peritonitis incidence and mortality: A meta-analysis. Dig Liver Dis 2016;48:353-9. [Crossref] [PubMed]
- Khan MA, Kamal S, Khan S, et al. Systematic review and meta-analysis of the possible association between pharmacological gastric acid suppression and spontaneous bacterial peritonitis. Eur J Gastroenterol Hepatol 2015;27:1327-36. [Crossref] [PubMed]
- Cichoż-Lach H, Michalak A. Current pathogenetic aspects of hepatic encephalopathy and noncirrhotic hyperammonemic encephalopathy. World J Gastroenterol 2013;19:26-34. [Crossref] [PubMed]
- Gupta A, Dhiman RK, Kumari S, et al. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol 2010;53:849-55. [Crossref] [PubMed]
- Sanaka M, Yamamoto T, Kuyama Y. Effects of proton pump inhibitors on gastric emptying: a systematic review. Dig Dis Sci 2010;55:2431-40. [Crossref] [PubMed]
- Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, iron, and magnesium. Curr Gastroenterol Rep 2010;12:448-57. [Crossref] [PubMed]
- Takeda Y, Doyama H, Tsuji K, et al. Does long-term use of proton pump inhibitors cause hypomagnesaemia in Japanese outpatients? BMJ Open Gastroenterol 2015;1:e000003. [Crossref] [PubMed]
- O'Leary F, Allman-Farinelli M, Samman S. Vitamin B12 status, cognitive decline and dementia: a systematic review of prospective cohort studies. Br J Nutr 2012;108:1948-61. [Crossref] [PubMed]
- Haenisch B, von Holt K, Wiese B, et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur Arch Psychiatry Clin Neurosci 2015;265:419-28. [Crossref] [PubMed]
- Gomm W, von Holt K, Thomé F, et al. Association of Proton Pump Inhibitors With Risk of Dementia: A Pharmacoepidemiological Claims Data Analysis. JAMA Neurol 2016;73:410-6. [Crossref] [PubMed]
- Lin ZN, Zuo YQ, Hu P. Association of Proton Pump Inhibitor Therapy with Hepatic Encephalopathy in Hepatitis B Virus-related Acute-on-Chronic Liver Failure. Hepat Mon 2014;14:e16258. [Crossref] [PubMed]
- Dam G, Vilstrup H, Watson H, et al. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology 2016;64:1265-72. [Crossref] [PubMed]
Cite this article as: Zhu J, Yu H, Mancuso A, Qi X. Proton pump inhibitors in liver cirrhosis: a review of benefits and harms. AME Med J 2017;2:36.

