
International Early Lung Cancer Action Program: update on lung cancer screening and the management of CT screen-detected findings
Introduction
CT screening was first introduced when helical CT scanners became available in the early 1990’s (1-5). Since then, there have been remarkable advances in CT scanner technology with concurrent increase in the number of CT examinations per year by approximately 10% annually (6). More powerful hardware and image reconstruction algorithms have allowed for faster scanning at lower radiation doses in today’s multidetector CT (MDCT) scanners. Regarding lung cancer screening, thinner collimation on modern CT scanners has led to the detection of smaller pulmonary nodules. Along with advances in imaging, there have been evolutions in diagnostic techniques such as percutaneous biopsies, navigational bronchoscopies, and PET scans. As these advances have altered clinical care, they have been integrated into the ongoing screening program.
The goal of any lung screening program is to find lung cancer as early as possible. However, in the context of screening asymptomatic individuals, unnecessary interventions that do not lead to the diagnosis of an invasive lung cancer will decrease the value of screening. Thus, there needs to be a balance between the goal of finding the cancer as early as possible and limiting unnecessary tests, especially invasive ones. Ultimately, this requires constant updating of the entire screening process.
Critical questions for any screening program are:
- Who should be screened, in other words, what are the indications for screening?
- How frequently should screening be performed?
- What is the optimal screening regimen?
The answers to these questions determine the effectiveness of the screening program, as well as its costs. Also, considerations need to be taken as to how the results are communicated to the referring physician and to the screening participant, typically with a lay summary provided to the latter.
Smoking cessation is a vital component of the screening program, not only for current smokers but also for former smokers to prevent relapse. CT screening provides a “teachable moment” for smoking cessation advice and has been shown to have no association with former smokers restarting the habit (7,8). Personalized counseling or referral to “Quit Smoking Help Lines” and other support groups are useful in helping smokers quit or preventing relapse thus such programs should be available.
The Early Lung Cancer Action Program (ELCAP) was created in New York in 1992 to assess the benefit of annual CT screening for lung cancer. ELCAP demonstrated that a high proportion of patients with lung cancer were diagnosed in Stage I utilizing CT, with a shift to smaller tumor sizes on annual repeat screening studies (2,3). Initial results were widely publicized and stimulated debate about the benefits of lung cancer screening. ELCAP has grown to involve multiple international institutions utilizing a common screening protocol, thereby creating the powerful International (I)-ELCAP prospectively acquired cohort.
Indications for screening
Approximately 222,500 new cases of lung cancer will be diagnosed in 2017, including about 44,500 in never smokers (9). Prior to the introduction of CT screening, less than 15% of newly diagnosed patients had Stage I disease. In comparison, studies on CT screening for lung cancer have shown that more than 80% of patients could be identified in clinical stage I (2-6,10-12). Other studies have shown similarly high rates of stage I diagnoses (13,14).
Screening is indicated for asymptomatic persons who are free of potential manifestations of lung cancer. Symptoms of lung cancer include worsening cough with hoarseness or hemoptysis, and unexplained weight loss. Individuals with these symptoms need a clinical workup, which should include a diagnostic CT of the chest. Of note, there used to be an obvious difference in the image quality between diagnostic CT scans and low dose screening CTs, but advancing technologies, like iterative reconstruction, have made this less of an issue. There remains, however, a difference in radiation dose, with a typical diagnostic CT being 10–20 times higher in dose than a low dose screening CT.
The United States Preventative Services Task Force (USPSTF) recommends CT screening for high-risk people aged 55 to 80 (15). It based its eligibility criteria on the National Lung Screening Trial (NLST) (16). The NLST studied adults 55 to 80 years old who were current smokers or quit in the past 15 years, and had a 30 or greater pack-year smoking history. Although the purpose of the NLST criteria to enroll high risk participants was to maximize the number of cancers diagnosed during the trial, these same criteria were adopted by the Centers for Medicare and Medicaid Services (CMS) as who should be screened (17). These CMS criteria state that screening should be discontinued if one has not smoked for more than 15 years or has developed a health problem that greatly reduces life expectancy or the ability or willingness to have curative lung surgery. This requirement is a concern, as it has been well-established that the risk of lung cancer persists long after smoking cessation, and these individuals remain at high risk (18).
Screening is recommended for high-risk individuals for whom the potential benefits of LDCT outweigh the risks. Current definitions of “high-risk” individuals rely on age and pack-years of smoking. The use of pack-years should be reconsidered as it is well known that duration and intensity of smoking are independent risk factors that should be considered separately, while pack-years of smoking combines these two factors (19).
The USPSTF criteria dichotomize the continuous variables of age and smoking history and are thus limited for determining risk of individuals. Also, the fact that 85% of heavy smokers will not develop lung cancer suggests that additional risk factors beyond age and smoking history should be included in order to more accurately identify individuals at high risk of lung cancer. Asbestos exposure is an example of an occupational risk factor (20) which markedly increases the risk of lung cancer. There are also other identifiable occupational and clinical risk factors that might be considered. Risk prediction models using additional risk factors seem promising, and as more information regarding their specificity and sensitivity emerges, such models may provide a better basis for selecting individuals at high-risk of lung cancer for screening.
Various analytic models have been developed to address the multitude of individual risk factors for developing lung cancer. The Bach model was developed to estimate the absolute risk that one will be diagnosed with lung cancer within 10 years (21). This model considers multiple risk factors: age, sex, prior history of asbestos exposure, smoking duration, average amount smoked per day, and time since smoking cessation for former smokers. The Bach model proved to be more sensitive than the NLST criteria of age and pack-years of smoking for predicting lung cancer incidence (80% vs. 71.4%), without loss of specificity (22,23).
Another model utilizing the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial database expanded the risk factors by including: gender, race, ethnicity, education, body mass index (BMI), COPD, emphysema, personal cancer history, personal pneumonia history, and family history of lung cancer (24). Unlike the NLST, the PLCO model did not exclude smokers based on pack-years or quit time. However, similarly to the NLST, it only recruited individuals 55 to 74 years of age. Study results showed that PLCO model had greater sensitivity (83% vs. 71.4%) without loss of specificity when compared to NLST criteria.
Current research is looking into strengthening this idea that risk-based models are crucial for determining the screening population. A 2017 retrospective analysis focused on nine previously established risk models (including the Bach and PLCO Models) and compared them to the NLST, to show that selection of the lung screening population using individual risk factors is superior to selection criteria of age and pack-years alone (23). Recent empirical modeling studies have been able to validate these risk-based models and show their relative effectiveness by estimating that they are associated with a greater number of lung cancer deaths prevented over five years, along with a lower “number needed to screen” to prevent one lung cancer death (25).
Utilizing risk prediction models to identify these “high-risk” individuals could increase cost-effectiveness and enhance screening efficiency by improving screening selection. Risk prediction models could also improve the shared decision-making process for patients and clinicians through easily accessible online risk calculators. Ultimately, more research on these models needs to be done.
Frequency of screening
There is a complex relationship between screening frequency and healthcare costs. A decrease in the frequency of screening with a loss in effectiveness will markedly reduce the value of the screening program. For example, a change from annual to biannual screening may cut the costs in half, but in turn can increase the frequency of higher stage cancer diagnoses, which then increases the cost of lung cancer treatment potentially offsetting the savings provided by the reduced frequency of screening.
In designing ELCAP, the decision to provide annual screening was based on the estimated growth rates of lung cancers (26-31). Growth rates were initially based principally on chest radiograph findings which did not allow for very accurate measurements. Typically reported volume doubling times (VDTs) of lung cancers were from 100 to 200 days. Since those early reports, studies have shown median growth rates of lung cancers to be about 120 to 180 days. Thus, a lung cancer with a doubling time of 122 days that is just becoming visible at 2 mm in diameter would grow to 4 mm in one year, and thus become readily detectable. To illustrate the importance of the doubling times, we give an example of an aggressive cancer and a much less aggressive one. A 2 mm lung cancer with a doubling time of 30 days, a typical small-cell lung cancer, would grow from 2 to 30 mm in one year, and most likely will no longer be a potentially curable Stage I lung cancer (11,32). On the other hand, a 2 mm lung cancer with a 400 days doubling time would grow to measure 2.5 mm one year later.
Some studies have looked into screening with different intervals between baseline and follow up scans, and screening intervals have been modeled by Duffy et al. (33) and Yankelevitz et al. (34). For example, the NELSON study (35) performed their first repeat screening one year after the baseline round, while the second and third scans were performed 2 and 2.5 years, respectively after the initial repeat round. In the second and third rounds of screening, they reported a lower frequency of diagnoses of stage I lung cancer, along with a higher frequency of interim cancers. Interim cancers are cases of lung cancer prompted by symptoms and diagnosed in between rounds of screening. As the time between screening rounds increases, the frequency of interim cancers typically increases. As one can see, the discussion of screening frequency requires a balance between minimizing the cost of the screening and maximizing the detection of early, curable lung cancers.
To date, the United States Preventive Services Task Force recommends annual screening (15) and, as a result, the CMS has mandated annual screening (17).
Regimen of screening
CT screening refers to the entire process of the pursuit of early, rule-in diagnoses of lung cancer. It begins with the initial, baseline low-dose CT scan and continues with repeat screenings. A positive result of each round of screening is followed by diagnostics using a well-defined algorithm. As the frequencies of different cell-types of lung cancer in the baseline round are different from those diagnosed in repeat rounds of screening, the definition of a positive result and the subsequent algorithm are defined separately for the baseline and subsequent annual repeat rounds (36). It is also understood that there may need to be occasional exceptions to the protocol based on clinical and imaging findings.
When the algorithm is applied in each screening round and does not lead to the diagnosis of malignancy, the next repeat screening is scheduled at a preset time. While the regimen has been continuously updated and improved by integrating new technologies and knowledge based on accrued screening results, its basic structure has remained unchanged.
The importance of a carefully defined screening regimen was highlighted in the comparison of I-ELCAP results and NLST results (37). The former used a defined algorithm, while the latter specifically stated that it did not specify one. I-ELCAP had a higher frequency of stage I lung cancer diagnoses (82% vs. 62%, P<0.0001) and a smaller median cancer size (17 vs. 23 mm, P<0.0001). Other subsequent screening studies have reported similar stage I results as I-ELCAP (12,33).
The nodule definitions and size thresholds of positive results have been continually reevaluated and updated. In the initial CT screening study (2,3), there was no size cutoff for positive results. Since then, updated thresholds have been introduced because of new CT scanner technology and accumulating evidence. The threshold for positive results in baseline screening was changed to 5 mm (38), and then eventually revised to 6 mm (38-40). Other organizations’ guidelines have followed suit and are now using the 6 mm threshold for baseline screening (41,42).
It was also shown that some solid and many subsolid pulmonary nodules resolved spontaneously, particularly new ones identified on repeat screening examinations (43). More than 70% of new nodules identified on annual repeat screening studies resolved by the time of the 1 month follow-up CT. Thus, follow-up imaging 3 months after baseline or 1 month after annual repeat screening is useful to avoid unnecessary diagnostic interventions, especially invasive diagnostic interventions.
Image acquisition
The low dose CT imaging technique remains consistent between baseline and repeat screenings. Given that there are a large variety of CT manufacturers and scanner models with high-resolution capabilities, the following are general guidelines for image production. Scans should be acquired on MDCTs, ideally with scanners having more than 16 rows. Images should be acquired so that scans can be reconstructed with a slice thickness of 1 mm or less. Studies have shown that thinner slices are better for automated image processing and nodule detection.
There is no specific definition of “low-dose”, although historically most screening protocols have used scan parameters of 120–140 kVp and 30–100 mAs. I-ELCAP experience suggests that scans be obtained at 120 kVp or lower and 40 mAs (effective) or lower. An alternative is to use dose-modulation, which should be established to correspond to approximately the same dose without modulation. Collimation and pitch also affect the dose, and these should be set to allow for the lowest possible dose while maintaining acceptable image quality. Image reconstruction should be performed using a standard, non-edge enhancing kernel to minimize the effects of noise. However, additional reconstructions may also be obtained, including maximum intensity projection (MIP) images. Scan parameters should also be adjusted to allow for patients with different body sizes. Dose modulation techniques that adjust for body size are available on most modern scanners, but if not, then scan parameters should be configured based on patient weight or BMI. Additionally, scan manufacturers are offering new dose reduction techniques, and their use is encouraged provided that acceptable image quality is maintained. Guidance on scan parameters specific to manufacturer make and model can be found on the website of the American Association of Physicists in Medicine (44).
Images should be acquired in a single breath from the lung apices through the lung bases. Standards should be established to ensure consistent breath holding. It is important to note that contrast material is not used. Just prior to performing the low-dose CT scan, the participant is asked to cough vigorously several times to clear the trachea and major bronchi of possible mucus secretions, thereby avoiding additional imaging that might be required to distinguish such secretions from endobronchial lesions. Any follow-up imaging of abnormalities identified in screening studies should be performed using the same low dose parameters, without contrast material, that are used for the baseline and repeat screenings.
Reading of images
The reader should be aware of the round (baseline or repeat) from which the images derives, as the work-up protocol depends on the specific screening round. The images are viewed on high-resolution monitors at their typical window and level settings—scrolling through the images one at a time. For the purposes of assessing the size of a nodule or that of a mediastinal abnormality, however, the following settings are typically used: lung window width 1,500 HU and lung window level 650 HU, and mediastinal window width 350 HU and mediastinal window level 25 HU.
Definitions of nodules, nodule consistency, and size
In both baseline and repeat screening, the reader’s primary concern is to identify and document all the visualized non-calcified nodules (NCNs). For repeat screenings, the reader’s goal is to identify any new NCNs and to discern whether any of the baseline nodules changed (e.g., growth or development of a solid component in a nonsolid nodule). To determine whether growth has occurred, the reader carefully compares the current images with the corresponding previous ones, displayed side-by-side.
For each parenchymal or endobronchial nodules, the reader documents location, size, consistency (solid, part-solid or nonsolid), edge characteristics (smooth or irregular), and presence/absence of calcification. The detailed definitions of nodules and their consistency and size are given below, followed by the assessment of nodule growth.
A nodule is defined as a focal non-linear opacity with a generally spherical shape surrounded by lung parenchyma. The nodule is classified as a NCN if it fails to meet the usual criteria for benign, calcified nodules. Thus, a nodule less than 6 mm in diameter is non-calcified if all of it appears less dense than the ribs (on bone and lung windows). A nodule measuring 6–20 mm in diameter is an NCN if most of it is non-calcified (by the above criterion) and/or the calcification does not correspond to a classical benign pattern (complete, central, lamellated, popcorn). A nodule measuring over 20 mm in diameter is considered a NCN if any part of it is non-calcified by the above criteria. Focal pleural thickening or pleural plaques are not considered nodules. Nodules of 30 mm or more are designated as masses.
A nodule is classified as solid unless it has specific characteristics to be classified as subsolid (45-51):
- Solid nodules with external or internal cystic airspaces or internal cavitation are still classified as solid nodules;
- Subsolid nodules may be either nonsolid or part-solid. A part-solid nodule is one whose solid internal components completely obscure the lung parenchyma, while a nonsolid nodule has none of its lung parenchyma completely obscured, allowing for visualization of background pulmonary vessels. Of note, in making the distinction between part-solid and nonsolid nodules, blood vessels within the nodule are not considered be considered to be solid components, despite their solid appearance. Lung cancers manifesting as part-solid nodules typically start as nonsolid nodules and subsequently develop a solid component (47).
When it is difficult to distinguish between part-solid and solid nodules, the nodule should be classified as solid. Nodules should also be classified as solid when the progression of a part-solid from a nonsolid nodule cannot be confirmed by review of prior images, particularly when the diameter of the solid component relative to the diameter of the entire nodule is 80% or larger. These definitions and recommendations are based on the radiologic findings of two large databases (46-49), recent systematic literature reviews (50,51) and also the current pathology literature (52,53).
Nodule size is reported according to its average diameter, which is the average of its length and width, that is (length + width)/2. Length and width are measured on a single CT image (axial, sagittal, or coronal) which shows the maximum size of the nodule. Length is the longest dimension of the nodule. Width, defined as the longest dimension perpendicular to the length, is measured on the same image.
For part-solid nodules, the focus is on the diameter of the solid component which is measured in the same way as solid nodules. Nonsolid nodules have no solid component.
The measured diameters should be supplemented by computer-based assessments of volume, but these techniques are still considered experimental and there is considerable variation in the volumetric measurements, particularly for complex nodules (27,28,54-56). In the future, when there is more sufficient evidence of its validity, volumetric analyses will likely replace manual diameter measurements.
Probability of lung cancer by nodule size and consistency
Figure 1 shows the probability of diagnosing lung cancer by nodule size and consistency in the baseline round of screening. The frequency of malignancy by nodule size is different in the baseline round than in annual repeat rounds, which are shown in Figure 2. For smaller sized nodules, the probability of malignancy is higher on annual repeat screening than on baseline screening. Also the probability of malignancy is lower for the larger size nodules on annual repeat screening. The actual number of cancers, especially among nonsolid nodules, cannot be fully addressed as pathologic diagnoses were not pursued in all cases.
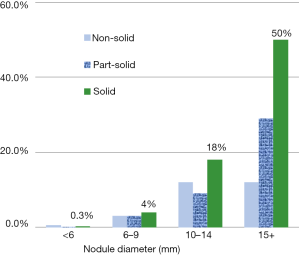
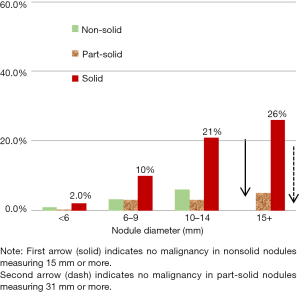
Based on review of the I-ELCAP experience over the past 20 years, there was no diagnosis of malignancy on annual repeat rounds in new nonsolid nodules greater than 15 mm or in part-solid nodules greater than 31 mm (46,47).
Positive results of baseline and annual repeat rounds of screening
The definition of a positive result is critical, as this determines the NCNs that are suspicious for lung cancer, and require further workup with biopsy or PET scans.
Baseline screening round: positive result definition
- The largest solid or solid component of part-solid NCN is 15 mm or larger in average diameter, or;
- If the largest solid or solid component of part-solid NCN is 6.0 to 14.9 mm and has showed growth at a malignant rate on 3 months follow-up low-dose CT scan (see growth assessment), or;
- Solid endobronchial nodule measuring 6.0 mm or larger which persists or grows on follow-up CT 3 months later.
Follow-up options for positive results on baseline are:
- If the nodule appearance is highly suggestive of lung cancer, biopsy is recommended.
- Another option is to perform a PET scan, particularly if the solid component of the nodule is 10 or more mm in diameter. If the PET result is positive, a biopsy is recommended, but if negative or indeterminate a low-dose CT is performed 1–3 months. If there is growth, biopsy is recommended, but if there is partial or complete resolution on the CT, the workup stops.
- If a solid endobronchial nodule has not decreased on the follow-up CT scan 3 months later, the participant is referred for pulmonary consultation, and if necessary, bronchoscopy.
All participants for whom the biopsy (considered to be adequate) did not lead to a diagnosis of lung cancer, a repeat CT 12 months after the initial baseline CT is to be performed.
Annual repeat rounds: positive result definition
- Largest new or growing solid or solid component of a part-solid nodule is 3.0–6.0 mm growing at a malignant rate on 6 month follow-up low-dose CT, or
- Largest new or growing solid or solid component of a part-solid nodule is 6.0 or larger growing at a malignant rate on 1 month follow-up low-dose CT, or
- New solid endobronchial nodule.
Follow-up options for positive results on annual repeat are:
- If the solid nodule or component of any newly identified part-solid nodule shows further growth at a malignant rate (Tables 1,2
), biopsy is recommended. Table 1
Annual repeat rounds: for new nodules between 3.0 and 5.0 mmOriginal diameter (mm) Diameter in 6 months without measurement error (mm) Diameter in 6 months with measurement error (mm) 3.0 3.8 4.2 4.0 5.0 5.4 5.0 6.3 6.7 Table 2
Annual repeat rounds: for new nodules 6.0 mm or largerOriginal diameter (mm) Diameter in 1 month without measurement error (mm) Diameter in 1 month with measurement error (mm) 6.0 6.2 7.0 7.0 7.3 8.1 8.0 8.3 9.1 9.0 9.4 10.2 10.0 10.4 11.2 11.0 11.4 12.2 12.0 12.5 13.3 13.0 13.5 14.3 14.0 14.5 15.3 - Another option is to perform a PET scan, particularly if the solid component of the nodule is 10 or more mm in diameter. If the PET result is positive, biopsy is recommended, but if negative or indeterminate a low-dose CT 1–3 months later is performed.
- If a solid endobronchial nodule has not decreased on the follow-up CT scan 1 month later, the participant is referred for pulmonary consultation and, if necessary, bronchoscopy.
All results that are not positive are considered to be negative or semi-positive and the participant is scheduled for the next annual repeat CT scan one year after the prior screening. This includes participants with multiple nodules for whom an occult infection or inflammation is a possibility. Such participants could be given a course of broad spectrum antibiotic with anaerobic coverage prior to the 1-month follow-up CT scan. If a solid endobronchial nodule is identified at the time of the initial CT, the participant is asked to cough vigorously several times and the region of interest is reimaged at that time. However, if classic features of retained secretions are identified such as low attenuation, air bubbles, stranding and multiplicity, no follow-up is needed.
Other organizations have guidelines that define a positive result differently, such as the American College of Radiology (41) and the National Comprehensive Cancer Network (42). Critical to the development of the I-ELCAP protocol has been the reliance on evidence obtained from its large well-documented database.
Assessment of growth
Growth of a nodule is defined as:
- Enlargement of the overall nodule size, regardless of consistency;
- Growth of the solid component of a part-solid nodule;
- Development of a solid component within a nonsolid nodule and;
- Increased attenuation of the nonsolid components within a nonsolid nodule.
Nodule VDTs are useful measures of malignancy and its aggressiveness (27,28,54-57). VDTs of less than 30 days are more suggestive of an infection than malignancy. Lung cancer VDTs are more than 30 days, typically between 30 and 400 days. VDTs can be based on the change in the nodule length, width, and height or on changes in direct volume measurements. However, volumetric measurements are complex and influenced by multiple factors including the intrinsic properties of the nodule and the software used to make the measurement (58). Additionally, they are impacted by the variability of CT scanners and their adjustable scan parameters (59-61).
Several groups have developed approaches to incorporate measurement errors into the determination of growth. The RSNA’s Quantitative Imaging Biomarkers Alliance (QIBA) (59) has developed a web-based calculator available at http://accumetra.com/solutions/qiba-lung-nodule-calculator. The American College of Radiology (ACR) specifies that growth for a nodule of any size requires “an increase of 1.5 mm or more” (41). Both the QIBA and ACR approaches allow for large degrees of measurement error to cover a wide range of CT scanners and the protocols.
I-ELCAP guidelines for solid nodules and the solid component of part-solid nodules are given in Tables 1-3. The tables assume that modern scan protocols and software are used to acquire the images at sub-pixel resolution. The assumptions are: (I) sub-millimeter CT slice thickness; (II) slice spacing equal to or less than slice thickness; (III) 64-detector-row or higher CT scanners; (IV) reconstruction field of view that is less than 30 cm; and (V) identical parameters on both scans.
Table 3
| Original diameter (mm) | Diameter in 3 months without measurement error (VDT: 180 days) | Diameter in 3 months with measurement error (VDT: 180 days) |
|---|---|---|
| 6.0 | 6.7 | 7.1 |
| 7.0 | 7.9 | 8.3 |
| 8.0 | 9.0 | 9.4 |
| 9.0 | 10.1 | 10.5 |
| 10.0 | 11.2 | 11.6 |
| 11.0 | 12.3 | 12.7 |
| 12.0 | 13.5 | 13.9 |
| 13.0 | 14.6 | 15.0 |
| 14.0 | 15.7 | 16.1 |
The shorter the time between CT scans (e.g., 1 month interval after the annual screening) the greater the impact of the measurement error, as the measurement error itself is greater. VDT, volume doubling time.
For solid nodules with little or no attachment to surrounding structures or for the solid component of part-solid nodules, the diameter change for a cancer with a VDT of 180 days is given in Tables 1-3. The first column gives the change in the nodule diameter (average of length and width) for VDTs of 180 days when there is no measurement error. The second column gives the diameter which must be exceeded when accounting for measurement error. Linear interpolation should be used for values in between the tabled values.
Computer-assisted evaluation of growth rates and VDTs is an active area of research. It should only be used in correlation with modern scanners and high-resolution protocols. With the careful technical and clinical quality review outlined below, the results of computer-assisted evaluation can be useful in the work-up for nodules:
- The computer scans and the segmentation should be inspected for image quality (e.g., motion artifacts) and for the quality of the segmentation;
- The radiologist should visually inspect both nodule image sets side-by-side to verify the quality of the computer segmentation for each image that contains a portion of the nodule;
- The segmentations should also be examined for errors such as when a vessel is segmented as part of a nodule in one scan but not in the other;
- Scan slice thickness for the purpose of volumetric analysis should not exceed 1.0 mm.
When using any computer-assisted software, the radiologist must be satisfied with the CT image quality and the computer segmentation results, further substantiating the notion that the decision of whether growth has occurred is ultimately based on clinical judgment.
For now, these guidelines on growth are meant to provide direction on whether nodule change has occurred qualitatively, as they do not prove accurate metrics regarding the quantitative rate of growth. There is ongoing research regarding confidence intervals for determining malignant growth rates within specified time intervals. Currently, any estimates of growth rates (or VDTs) should be interpreted with caution.
Once growth at a malignant rate is documented, biopsy is recommended prior to treatment. Linek et al. showed the usefulness of percutaneous biopsy to limit surgical resection of benign nodules (62).
Other thoracic and abdominal findings and follow-up recommendations
The radiologist is also responsible for documenting other findings in the thorax, including those visualized within the mediastinum, heart, breasts, overlying soft tissues, abdomen, and bones. In an optimal screening program, close collaboration with the relevant medical subspecialties is recommended for appropriate follow-up.
Discrete cystic airspaces
The walls of discrete cystic airspaces (e.g., air cysts) should be assessed for progressive wall thickening, both in increasing thickness and increasing circumferential wall involvement, as these may be due to lung cancer (63).
Emphysema
The extent of emphysema is identified and classified as none, mild, moderate, or severe, each being scored quantitatively from 0 to 3, respectively. Mild emphysema is defined by having no discrete areas of decreased CT attenuation, but splaying of blood vessels, suggesting parenchymal expansion, or occasional discrete areas of decreased attenuation; moderate emphysema if discrete areas of decreased attenuation can be identified involving less than half of the lung parenchyma; and, severe emphysema if discrete areas of decreased attenuation can be identified involving more than half of the lung parenchyma. Subsequently, each subject receives an emphysema score in the range of 0 to 3 (64). If emphysema is present and was previously unrecognized, consultation with a pulmonologist is recommended. Also airway wall thickness can be identified by the radiologist or computer algorithms (65).
Interstitial findings
Interstitial lung diseases can be identified in asymptomatic screening patients. Differentiation between the various interstitial diseases is important, particularly with usual interstitial pneumonia (UIP), which carries a worse prognosis and requires a different treatment regimen. Findings of UIP have been classified between pre-honeycomb (HC) and HC findings (66). Other interstitial diseases such as non-specific interstitial pneumonia (NSIP) or hypersensitivity pneumonitis (HP) may differ in location and radiographic findings (67). Pre-honeycomb findings may start with traction bronchiectasis or bronchiolectasis and then progress to include ground-glass opacities and reticulations, typically at the periphery of the lungs and with a basilar predominant distribution. The likelihood of disease progression is associated with the presence of honeycombing. Early identification is important and consultation with a pulmonologist is recommended the above findings are identified.
Mediastinal and thymic masses
Masses can occur anywhere in the mediastinum, including in the thymus, heart, and esophagus. Masses in the neck or thyroid may also extend inferiorly through the thoracic inlet into the mediastinum. On these non-contrast screening studies, the size, margins, density, and precise location of these masses should be documented.
Based on the frequency and natural course of thymic masses identified in baseline and annual repeat screenings for lung cancer (68), the following work-up recommendations are made: If the lesion is 3.0 cm or less in diameter on baseline CT without invasive features (e.g., irregular borders or loss of fat planes), follow-up CT one year later is recommended. If the thymic mass is greater than 3.0 cm or shows growth on the follow-up CT, further workup according to standard practice is recommended with surgical and pulmonology consultation.
Coronary arteries
Each coronary artery is identified (left main, left anterior descending, circumflex, and right coronary artery). Evidence of calcification in each artery is documented as none, minimal, moderate, or severe, scored as 0, 1, 2, and 3, respectively. Minimal calcification is defined if less than 1/3 of the length of the entire artery is calcified, moderate if 1/3–2/3 is calcified, and severe if more than 2/3 shows calcification. With four arteries thus scored, each subject received an Ordinal Coronary Artery Calcium (CAC) Score in the range from 0 to 12 and the corresponding recommendations are given in the section on the workup of ancillary findings (68-72). Currently, it is also possible to obtain the Agatston, volume or mass calcium scores on low-dose CT scans and 3.0 mm CT scans can be obtained which are needed to determine the standard Agatston score. New rapid scanning techniques minimize cardiac motion and allow for improved Agatston scoring on non-gated examinations. The equivalence of these scores to standard dose gated scanning is still being established. Of note, it is important to distinguish between coronary artery calcium and coronary artery stents, which also appear dense/calcified. The thinner slice series are generally better at differentiating between the two. Table 4 gives the Ordinal CAC Score, the equivalent Agatston Score, and recommendations.
Table 4
| Ordinal CAC score | Agatston score | Recommendation |
|---|---|---|
| 0 | 0 | Probability of cardiovascular heart disease (CHD) is low. Reassure and keep healthy lifestyle |
| 1–3 | 1–100 | Probability of CHD is mild to moderately increased; healthy lifestyle; moderate statin; ASA |
| 4–12 | > 100 | Probability of CHD is moderate to high. Healthy lifestyle; very intensive statin + second drug as needed; ASA; Consider function testing to r/o obstruction; Aggressive BP lowering; Referral to internist or preventive cardiologist |
CAC, Coronary Artery Calcification; ASA, acetylsalicylic acid; r/o, rule out.
The recommendations for Ordinal Score are based on prior analyses of screening data (69-73). Additional analysis showed there is excellent agreement in the Ordinal CAC Score for the categories of the Agatston Scores. Latest recommendations are detailed in SCCT/STR guidelines (74).
Breast density
Using mediastinal windows, the CT images of the breast are reviewed and classified according to the Breast Imaging Reporting and Data System (BI-RADS) developed by the American College of Radiology (75). The BI-RADS classification identifies four grades according to the breast density.
- Grade 1: almost entirely fatty.
- Grade 2: there are scattered fibroglandular densities.
- Grade 3: breasts are heterogeneously dense, which may obscure small masses.
- Grade 4: breasts are extremely dense, which lowers the sensitivity of mammography.
The key differentiation is between Grades 1–2 and 3–4 (74,76). A Grade of 3 or 4 should be specifically noted in the report, as it suggests an increased risk for breast cancer and, if clinically indicated, ultrasound (77) or MRI (78) of the breast is suggested as mammography may miss an early cancer or precursor lesion.
Adrenal enlargement
When there is thickening or nodular enlargement of either adrenal gland measuring 40 mm or more in the largest transverse diameter, further evaluation is recommended (79). Adrenal enlargement of less than 40 mm in transverse diameter and low attention (less than 10 HU) can be followed by annual low-dose CT scans until growth is identified.
Liver steatosis
If liver attenuation is below 40 HU and/or the liver-spleen ratio is below 0.9, then we recommend follow-up with a primary care physician or liver specialist for further evaluation of possible hepatic steatosis (80).
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ricardo Sales dos Santos, Myrna Godoy, Juliana Franceschini and Hiran C. Fernando) for the series “Update on Lung Cancer Screening and the Management of CT Screening Detected Pulmonary Nodules” published in AME Medical Journal. The article has undergone external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.08.24). The series “Update on Lung Cancer Screening and the Management of CT Screening Detected Pulmonary Nodules” was commissioned by the editorial office without any funding or sponsorship. Dr. Yankelevitz is a named inventor on a number of patents and patent applications relating to the evaluation of diseases of the chest including measurement of nodules. Some of these, which are owned by Cornell Research Foundation (CRF), are non-exclusively licensed to General Electric. As an inventor of these patents, Dr. Yankelevitz is entitled to a share of any compensation which CRF may receive from its commercialization of these patents. He is also an equity owner in Accumetra, a privately held technology company committed to improving the science and practice of image-based decision making. Dr. Yankelevitz also serves on the advisory board of GRAIL. Dr. Henschke is the President and serves on the board of the Early Diagnosis and Treatment Research Foundation. She receives no compensation from the Foundation. The Foundation is established to provide grants for projects, conferences, and public databases for research on early diagnosis and treatment of diseases. Dr. Henschke is also a named inventor on a number of patents and patent applications relating to the evaluation of pulmonary nodules on CT scans of the chest which are owned by Cornell Research Foundation (CRF). Since 2009, Dr. Henschke does not accept any financial benefit from these patents including royalties and any other proceeds related to the patents or patent applications owned by CRF. The authors have no other conflicts of interest to declare.
I-ELCAP Investigators: Mount Sinai School of Medicine, New York, NY: Claudia I. Henschke, Principal Investigator, David F. Yankelevitz, Rowena Yip, Dongming Xu, Mary Salvatore, Raja Flores, Andrea Wolf; Weill Cornell Medical College: Dorothy I. McCauley, Mildred Chen, Daniel M. Libby, James P. Smith, Mark Pasmantier; Cornell University: Anthony P. Reeves; CBNS, City University of New York at Queens College, Queens, NY: Steven Markowitz, Albert Miller; Fundacion Instituto Valenciano de Oncologia, Valencia, Spain: Jose Cervera Deval; University of Toronto, Princess Margaret Hospital, Toronto, Canada: Heidi Roberts, Demetris Patsios; Azumi General Hospital, Nagano, Japan: Shusuke Sone, Takaomi Hanaoka; Clinica Universitaria de Navarra, Pamplona, Spain: Javier Zulueta, Juan P. de-Torres, Maria D. Lozano; Swedish Medical Center, Seattle, WA: Ralph Aye, Kristin Manning; Christiana Care, Helen F. Graham Cancer Center, Newark, DE: Thomas Bauer; National Cancer Institute Regina Elena, Rome, Italy: Stefano Canitano, Salvatore Giunta; St. Agnes Cancer Center, Baltimore, MD: Enser Cole; LungenZentrum Hirslanden, Zurich, Switzerland: Karl Klingler; Columbia University Medical Center, New York, NY: John H. M. Austin, Gregory D. N. Pearson; Hadassah Medical Organization, Jerusalem, Israel: Dorith Shaham; Holy Cross Hospital Cancer Institute, Silver Spring, MD: Cheryl Aylesworth; Nebraska Methodist Hospital, Omaha NE: Patrick Meyers; South Nassau Communities Hospital, Long Island, NY: Shahriyour Andaz; Eisenhower Lucy Curci Cancer Center, Rancho Mirage, CA: Davood Vafai; New York University Medical Center, New York, NY: David Naidich, Georgeann McGuinness; Dorothy E. Schneider Cancer Center, Mills-Peninsula Health Services, San Mateo, CA: Barry Sheppard; State University of New York at Stony Brook, Stony Brook, NY: Matthew Rifkin; ProHealth Care Regional Cancer Center, Waukesha & Oconomowoc Memorial Hospitals, Oconomowoc, WI: M. Kristin Thorsen, Richard Hansen; Maimonides Medical Center, Brooklyn, NY: Samuel Kopel; Wellstar Health System, Marietta GA: William Mayfield; St. Joseph Health Center, St. Charles, MO: Dan Luedke; Roswell Park Cancer Institute, Buffalo, NY: Donald Klippenstein, Alan Litwin, Peter A. Loud; Upstate Medical Center, Syracuse, NY: Leslie J. Kohman, Ernest M. Scalzetti; Jackson Memorial Hospital, University of Miami, Miami, FL; Richard Thurer, Nestor Villamizar; State University of New York, North Shore-Long Island Jewish Health System, New Hyde Park, NY: Arfa Khan, Rakesh Shah; The 5th Affiliated Hospital of Sun Yat-Sen University, Zhuhai, China: Xueguo Liu; Mercy Medical Center, Rockville Center, NY: Gary Herzog; Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan: Diana Yeh; National Cancer Institute of China, Beijing, China: Ning Wu; Staten Island University Hospital, Staten Island NY: Joseph Lowry, Mary Salvatore; Central Main Medical Center: Carmine Frumiento; Mount Sinai School of Medicine, New York, NY: David S. Mendelson; Georgia Institute for Lung Cancer Research, Atlanta, GA: Michael V. Smith; The Valley Hospital Cancer Center, Paramus NJ: Robert Korst; Health Group Physimed/McGill University, Montreal, CA: Jana Taylor; Memorial Sloan-Kettering Cancer Center, New York, NY: Michelle S. Ginsberg; John Muir Cancer Institute, Concord CA: Michaela Straznicka; Atlantic Health Morristown Memorial Hospital, Morristown NJ: Mark Widmann; Alta Bates Summit Medical Center, Berkeley CA: Gary Cecchi; New York Medical College, Valhalla, NY: Terence A.S. Matalon; St. Joseph’s Hospital, Atlanta GA: Paul Scheinberg; Mount Sinai Comprehensive Cancer Center, Miami Beach, FL: Shari-Lynn Odzer; Aurora St. Luke’s Medical Center, Milwaukee WI: David Olsen; City of Hope National Medical Center, Duarte, CA: Fred Grannis, Arnold Rotter; Evanston Northwestern Healthcare Medical Group, Evanston, IL: Daniel Ray; Greenwich Hospital, Greenwich, CT: David Mullen; Our Lady of Mercy Medical Center, Bronx, NY: Peter H. Wiernik; Baylor University Medical Center, Dallas TX: Edson H. Cheung; Sequoia Hospital, Redwood City CA: Melissa Lim; Glens Falls Hospital, Glens Falls NY: Louis DeCunzo; Atlantic Medical Imaging, Atlantic City NJ: Robert Glassberg; Karmanos Cancer Institute, Detroit, MI: Harvey Pass, Carmen Endress; Rush University, Chicago IL: Mark Yoder, Palmi Shah; Building Trades, Oak Ridge TN: Laura Welch; Sharp Memorial Hospital, San Diego, CA: Michael Kalafer; Newark Beth Israel Medical Center, Newark NJ Jeremy Green; Guthrie Cancer Center, Sayre PA: James Walsh, David Bertsch; Comprehensive Cancer Centers of the Desert, Palm Springs CA: Elmer Camacho; Dickstein Cancer Treatment Center, White Plains Hospital, White Plains NY: Cynthia Chin; Presbyterian Healthcare, Charlotte NC: James O’Brien; University of Toledo, Toledo OH: James C. Willey.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Henschke CI, Miettinen OS, Yankelevitz DF, et al. Radiographic screening for cancer. Proposed paradigm for requisite research. Clin Imaging 1994;18:16-20. [Crossref] [PubMed]
- Henschke CI, McCauley D, Yankelevitz D, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99-105. [Crossref] [PubMed]
- Henschke CI, Naidich D, Yankelevitz D, et al. Early lung cancer action project: initial findings on repeat screenings. Cancer 2001;92:153-9. [Crossref] [PubMed]
- Kaneko M, Eguchi K, Ohmatsu H, et al. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology 1996;201:798-802. [Crossref] [PubMed]
- Sone S, Nakayama T, Honda T, et al. Long-term follow-up study of a population-based 1996-1998 mass screening programme for lung cancer using mobile low-dose spiral computed tomography. Lung Cancer 2007;58:329-41. [Crossref] [PubMed]
- Tabari A, Gullo RL, Murugan V, et al. Recent Advances in Computed Tomographic Technology. J Thorac Imaging 2017;32:89-100. [Crossref] [PubMed]
- Ostroff JS, Buckshee N, Mancuso C, et al. Smoking cessation following CT screening for early detection of lung cancer. Prev Med 2001;33:613-21. [Crossref] [PubMed]
- Anderson CM, Yip R, Henschke CI, et al. Smoking cessation and relapse during a lung cancer screening program. Cancer Epidemiol Biomarkers Prev 2009;18:3476-83. [Crossref] [PubMed]
- American Cancer Society (ACS). Cancer Facts & Figures 2017. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf
- Henschke CI, Wisnivesky JP, Yankelevitz DF, et al. Small stage I cancers of the lung: genuineness and curability. Lung Cancer 2003;39:327-30. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF, Miettinen OS, et al. Computed tomographic screening for lung cancer: the relationship of disease stage to tumor size. Arch Intern Med 2006;166:321-5. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators. Survival of Patients with Stage I lung cancer detected on CT screening. NEJM 2006;355:1763-71. [Crossref] [PubMed]
- Horeweg N, van der Aalst CM, Thunnissen E, et al. Characteristics of Lung Cancers Detected by Computer Tomography Screening in the Randomized NELSON Trial. Am J Respir Crit Care Med 2013;187:848-54. [Crossref] [PubMed]
- Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. [Crossref] [PubMed]
- Moyer VAU.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330-8. [PubMed]
- Aberle DR, Adams A, Berg C, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Centers for Medicare and Medicaid Services (CMS). Proposed Decision Memo for Screening for Lung Cancer with Low Dose Computed Tomography (LDCT) (CAG-00439N). Available online: http://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=274, January 2, 2015.
- Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst 1981;66:1191-308. [Crossref] [PubMed]
- Leffondré K, Abragamowicz M, Siemiatycki J, et al. Modeling Smoking History: A Comparison of Different Approaches. Am J Epidemiol 2002;156:813-23. [Crossref] [PubMed]
- Selikoff IJ, Hammond EC, Churg C. Asbestos Exposure, Smoking, and Neoplasia. JAMA 1968;204:106-12. [Crossref] [PubMed]
- Etzel CJ, Bach PB. Estimating Individual Risk for Lung Cancer. Semin Respir Crit Care Med 2011;32:3-9. [Crossref] [PubMed]
- Marcus MW, Raji OY, Field JK. Lung cancer screening: identifying the high risk cohort. J Thorac Dis 2015;7:S156-S162. [PubMed]
- Ten Haaf K, Jeon J, Tammemägi MC, et al. Risk prediction models for selection of lung cancer screening candidates: A retrospective validation study. PLoS Med 2017;14:e1002277. [Crossref] [PubMed]
- Tammemägi MC, Katki HA, Hocking WG, et al. Selection Criteria for Lung-Cancer Screening. N Engl J Med 2013;368:728-36. [Crossref] [PubMed]
- Katki HA, Kovalchik SA, Berg CD, et al. Development and Validation of Risk Models to Select Ever-Smokers for CT Lung Cancer Screening. JAMA 2016;315:2300-11. [Crossref] [PubMed]
- Flehinger BJ, Kimmel M, Melamed MR. The Effect of Surgical Treatment on Survival from Early Lung Cancer. Chest 1992;101:1013-8. [Crossref] [PubMed]
- Yankelevitz DF, Gupta R, Zhao B, et al. Small pulmonary nodules: evaluation with repeat CT--preliminary experience. Radiology 1999;212:561-6. [Crossref] [PubMed]
- Yankelevitz DF, Reeves AP, Kostis WJ, et al. Small pulmonary nodules: volumetrically determined growth rates based on CT evaluation. Radiology 2000;217:251-6. [Crossref] [PubMed]
- Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 2000;73:1252-9. [Crossref] [PubMed]
- Detterbeck FC, Marom EM, Arenberg DA, et al. The IASLC Lung Cancer Staging Project: Background Data and Proposals for the Application of TNM Staging Rules to Lung Cancer Presenting as Multiple Nodules with Ground Glass or Lepidic Features or a Pneumonic Type of Involvement in the Forthcoming Eighth Edition of the TNM Classification. J Thorac Oncol 2016;11:666-80.
- Henschke CI, Yankelevitz D, Yip R, et al. Lung cancers diagnosed at annual CT screening: volume doubling times. Radiology 2012;263:578-83. [Crossref] [PubMed]
- Austin JH, Yip R, D'Souza BM, et al. Small-cell carcinoma of the lung detected by CT screening: stage distribution and curability. Lung Cancer 2012;76:339-43. [Crossref] [PubMed]
- Duffy SW, Field JK, Allgood PC, et al. Translation of research results to simple estimates of the likely effect of a lung cancer screening programme in the United Kingdom. Br J Cancer 2014;110:1834-40. [Crossref] [PubMed]
- Yankelevitz D, Henschke C. Considerations for Determining the Interval Between Rounds of Low Dose CT Screening for Lung Cancer. Nat Rev Clinc Oncol 2016;13:533-4. [Crossref]
- Yousaf-Khan U, van der Aalst C, de Jong PA, et al. Final screening round of the NELSON lung cancer screening trial: the effect of a 2.5 screening interval. Thorax 2017;72:48-56. [Crossref] [PubMed]
- Henschke CI, Salvatore M, Cham M, et al. Baseline and annual repeat rounds of screening: implications for optimal regimens of screening. Eur Radiol 2017; [Crossref]
- Yip R, Henschke C, Yankelevitz D, et al. The impact of the regimen of screening on lung cancer cure: a comparison of I-ELCAP and NLST. Eur J Cancer Prev 2015;24:201-8. [Crossref] [PubMed]
- Henschke CI, Yankelevitz D, Naidich D, et al. CT screening for lung cancer: suspiciousness of nodules according to size on baseline scans. Radiology 2004;231:164-8. [Crossref] [PubMed]
- Henschke CI, Yip R, Yankelevitz D, et al. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2013;158:246-52. [Crossref] [PubMed]
- Yip R, Henschke CI, Yankelevitz DF, et al. CT screening for lung cancer: alternative definitions of positive test result based on the national lung screening trial and international early lung cancer action program databases. Radiology 2014;273:591-6. [Crossref] [PubMed]
- Kazerooni EA, Armstrong MR, Amorosa JK, et al. ACR CT accreditation program and the lung cancer screening program designation. J Am Coll Radiol 2015;12:38-42. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 4.2016. J Natl Compr Canc Netw 2016;14:255-64. [Crossref] [PubMed]
- Libby DM, Wu N, Lee IJ, et al. CT screening for lung cancer: the value of short-term CT follow-up. Chest 2006;129:1039-42. [Crossref] [PubMed]
- American Association of Physicists in Medicine. Lung Cancer Screening CT. Available online: http://www.aapm.org/pubs/CTProtocols/?tab=5#CTabbedPanels
- Henschke CI, Yankelevitz D, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-7. [Crossref] [PubMed]
- Yankelevitz DF, Yip R, Smith JP, et al. CT Screening for Lung Cancer: Nonsolid Nodules in Baseline and Annual Repeat Rounds. Radiology 2015;277:555-64. [Crossref] [PubMed]
- Henschke CI, Yip R, Wolf A, et al. CT screening for lung cancer: part-solid nodules in baseline and annual repeat rounds. AJR Am J Roentgenol 2016;207:1176-84. [Crossref] [PubMed]
- Yip R, Yankelevitz DF, Hu M, et al. Lung Cancer Deaths in the National Lung Screening Trial Attributed to Nonsolid Nodules. Radiology 2016;281:589-96. [Crossref] [PubMed]
- Yip R, Henschke C, Li K, et al. Lung Cancers Manifesting as Part-Solid Nodules in the National Lung Screening Trial. AJR Am J Roentgenol 2017;208:1011-21. [Crossref] [PubMed]
- Yip R, Wolf A, Tam K, et al. Outcomes of lung cancers manifesting as nonsolid nodules. Lung Cancer 2016;97:35-42. [Crossref] [PubMed]
- Yip R, Li K, Liu L, et al. Controversies on lung cancers manifesting as part-solid nodules: a systematic review. Eur Radiol 2017; [Crossref]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke A, et al. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus and heart. J Thorac Oncol 2015;10:1240-2. [Crossref] [PubMed]
- Kostis WJ, Reeves A, Yankelevitz D, et al. Three-dimensional segmentation and growth-rate estimation of small pulmonary nodules in helical CT images. IEEE Trans Med Imaging 2003;22:1259-74. [Crossref] [PubMed]
- Reeves AP, Chan A, Yankelevitz D, et al. On measuring the change in size of pulmonary nodules. IEEE Trans Med Imaging 2006;25:435-50. [Crossref] [PubMed]
- Kostis WJ, Yankelevitz D, Reeves A, et al. Small pulmonary nodules: reproducibility of three-dimensional volumetric measurement and estimation of time to follow-up CT. Radiology 2004;231:446-52. [Crossref] [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [Crossref] [PubMed]
- Liang M, Yip R, Tang W, et al. Variation of CT screen-detected nodule volumetry as a function of size. AJR Am J Roentgenol 2017;209:304-8. [Crossref] [PubMed]
- Radiological Society of North America. Quantitative Imaging Biomarker Alliance. Available online: http://accumetra.com/solutions/qiba-lung-nodule-calculator
- Armato SG 3rd, McLennan G, McNitt-Gray MF, et al. Lung image database consortium: developing a resource for the medical imaging research community. Radiology 2004;232:739-48. [Crossref] [PubMed]
- Henschke CI, Yankelevitz DF, Yip R, et al. Tumor volume measurement error using computed tomography (CT) imaging in a Phase II clinical trial in lung cancer. J Med Imaging (Bellingham) 2016;3:035505. [Crossref] [PubMed]
- Linek HC, Flores RM, Yip R, et al. Non-malignant resection rate is lower in patients who undergo pre-operative fine needle aspiration for diagnosis of suspected early-stage lung cancer. Am J Respir and Crit Care Med 2015;191:A3561.
- Farooqi AO, Cham M, Zhang L, et al. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol 2012;199:781-6. [Crossref] [PubMed]
- Zulueta JJ, Wisnivesky JP, Henschke CI, et al. Emphysema scores predict death from COPD and lung cancer. Chest 2012;141:1216-23. [Crossref] [PubMed]
- Lee J, Reeves AP, Yankelevitz D, et al. Skewness Reduction Approach for Measuring Airway Wall Thickness. Int J CARS 2008;3:S50-S52.
- Salvatore M, Henschke C, Yip R, et al. Evidence of Interstitial Lung Disease on Low-Dose Chest CT Images: Prevalence, Patterns, and Progression. AJR Am J Roentgenol 2016;206:487-94. [Crossref] [PubMed]
- Southern BD, Scheraga RG, Yadav R. Managing interstitial lung disease detected on CT during lung cancer screening. Cleve Clin J Med 2016;83:55-65. [Crossref] [PubMed]
- Henschke CI, Lee IJ, Wu N, et al. CT screening for lung cancer: prevalence and incidence of mediastinal masses. Radiology 2006;239:586-90. [Crossref] [PubMed]
- Shemesh J, Henschke CI, Farooqi A, et al. Frequency of coronary artery calcification on low-dose computed tomography screening for lung cancer. Clin Imaging 2006;30:181-5. [Crossref] [PubMed]
- Shemesh J, Henschke CI, Shaham D, et al. Ordinal scoring of coronary artery calcifications on low-dose CT scans of the chest is predictive of death from cardiovascular disease. Radiology 2010;257:541-8. [Crossref] [PubMed]
- Htwe Y, Cham M, Henschke C, et al. Coronary artery calcification on low-dose computed tomography: comparison of Agatston and Ordinal Scores. Clin Imaging 2015;39:799-802. [Crossref] [PubMed]
- Hecht HS, de Siqueira M, Cham M, et al. Low- vs. standard-dose coronary artery calcium scanning. Eur Heart J Cardiovasc Imaging 2015;16:358-63. [Crossref] [PubMed]
- Hecht HS, Cronin P, Blaha MJ, et al. 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. J Cardiovasc Comput Tomogr 2017;11:74-84. [Crossref] [PubMed]
- Salvatore M, Margolies L, Kale M, et al. Breast density: comparison of chest CT with mammography. Radiology 2014;270:67-73. [Crossref] [PubMed]
- Sickles EA, D’Orsi CJ, Bassett LW, et al. ACR BI-RADS® Mammography. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology, 2013.
- Margolies L, Salvatore M, Eber C, et al. The general radiologist's role in breast cancer risk assessment: breast density measurement on chest CT. Clin Imaging 2015;39:979-82. [Crossref] [PubMed]
- Mendelson EB, Böhm-Vélez M, Berg WA, et al. ACR BI-RADS Ultrasound. In: ACR BI-RADS Atlas, Breast Imaging Reporting and Data System, 5th Edition. Reston, VA, American College of Radiology, 2013:128-30.
- Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS® Magnetic Resonance Imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology, 2013.
- Hu M, Yip R, Yankelevitz DY, et al. CT screening for lung cancer: Frequency of enlarged adrenal glands identified in baseline and annual repeat rounds. Eur Radiol 2016;26:4475-81. [Crossref] [PubMed]
- Chen X, Li K, Yip R, et al. Hepatic steatosis in participants in a program of low-dose CT screening for lung cancer. Eur J Radiol 2017; [Crossref] [PubMed]
Cite this article as: Chung M, Tam K, Wallace C, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP Investigators. International Early Lung Cancer Action Program: update on lung cancer screening and the management of CT screen-detected findings. AME Med J 2017;2:129.

