
Trimethylamine N-oxide and platelets aggregation: insufficient evidence for causal inference in thrombosis
Trimethylamine N-oxide (TMAO) is an amine oxide generated in the liver from nutrients such as choline, betaine, or carnitine via an intermediate gut-bacteria driven metabolite, trimethylamine (TMA). Recently, Zhu et al. conducted a 2-month open-, non-placebo controlled intervention in vegetarians and omnivores using 450 mg total choline/day (1). Zhu et al. reported a significant increase in plasma TMAO concentrations (from 2.5 to 36.4 M in omnivores and from 2.6 to 27.2 M in vegetarians). A corresponding increase in platelet aggregation according to an in vitro platelets function test was also reported and expressed as percentage of maximal amplitude. The assumed effects of TMAO on platelets aggregation were observed at the first follow-up visit, 1 month after the start of supplementation, and the effects were stronger in omnivores compared with vegetarians (1). No further changes occurred in the following month of supplementation. There is no information on latency period, sustainability of the effect, or resistance to high TMAO levels. The results suggest that the increase in platelet aggregation have leveled off after 1 month (no further increase between month 1 and month 2). There is no evidence on sustainability of the effect after the last oral choline dose that was taken in the evening before the platelet function test. The results of the adenosine diphosphate (ADP)-induced platelets aggregation in vitro were interpreted as prothrombotic effect of TMAO (1). After aspirin usage in subjects without platelets disorders, lowering of in vitro platelets-reactivity to 5 M ADP was hypothesized to indicate that “TMAO may overcome antiplatelet effects of aspirin”. Nevertheless, the interactive effect of aspirin and TMAO can be equally argued to indicate that: “TMAO may reduce the risk of bleeding from aspirin” or “TMAO may reduce resistance to aspirin in subjects who need anti-platelet drugs”. But how to interpret the results in term of cause and effect?
Platelet aggregation is a highly complex process involving numerous cellular receptors and transmembrane pathways. Platelet activation occurs when agonists, such as ADP, thromboxane A2 (TxA2), and thrombin, bind to their receptors. This physiological process is involved in protective hemostasis (i.e., prevents bleeding by forming cloth) as well as in pathological thrombosis (over-aggregation). A variety of agonists such as ADP, epinephrine, arachidonic acid, or collagen can induce platelet aggregations via different mechanisms (2). This characteristic has been used for in-vitro diagnosis of platelet disorders and for monitoring resistance to anti-platelets. Nevertheless, assays that use a single agonist or a single concentration of any agonist are oversimplification of platelet function that could be completely different under physiological conditions (3).
In vivo platelets activation causes ADP to release from dense granules. ADP activates surface glycoprotein IIb/IIIa that is attached to fibrinogen, thus leading to aggregation of platelets to adherent layer. Adding ADP to platelets rich plasma (in vitro) causes an initial increase in aggregation due to activation of the glycoprotein IIb/IIIa platelets membrane receptor and a second wave of aggregation due to recruitment of additional platelets aggregates. In contrast, aspirin inhibits platelet activation mainly by targeting cyclooxygenase 1 (COX-1) thus leading to inhibition of TxA2 formation. Because arachidonic acid affects the COX-1/TxA2 system, this compound is used, instead of ADP, for in vitro induction of platelets aggregation in platelet rich plasma under aspirin treatment. Despite that aspirin has been shown to reduce platelet aggregation induced by ADP, aspirin inhibition of platelets aggregation after arachidonic acid is greater and this test is used for routine monitoring of aspirin effect (4,5).
Zhu et al. observed higher platelet aggregation at high TMAO (compared with low TMAO) and lower aggregation under aspirin compared with the same subject without aspirin (1). The results are not interpretable for the following reasons; first, because the results of the platelets aggregation in platelets rich plasma are not comparable between studies, agonists, and agonists concentrations (6); second, because TMAO was anticipated to inhibit surface glycoprotein IIb/IIIa (that is activated by ADP), but aspirin acts mainly via TxA2. Thus, using ADP as an agonist for the surrogate platelet aggregation test is not selective for aspirin effect. However, what would have happened in subjects with indication for anti-platelets treatment? Could high TMAO be protective against bleeding? Could it reduce resistance to long term antiplatelet therapy? Could there be a platelet-adaptation to high choline intake? Clearly these questions are not answered yet.
The long-term risk of thrombosis associated with high choline intake or high plasma TMAO is not evident. The value of platelet function tests in predicting future thrombosis in non-symptomatic individuals has been questioned by the Framingham Heart Study cohort where no association was found between several platelets function tests (including ADP-aggregation) and future thrombosis after controlling for other likely competing risk factors (6). Similar negative results were reported by Weber et al., who found that ADP-aggregation was not associated with thrombosis (7). Moreover, compared with omnivores, vegetarians could have fewer or larger platelets. In addition, any possible association between TMAO and platelet functions could be subject to effect modification from dietary components such as betaine, carnitine, fatty acids, lipids, or micronutrients (8,9). In line with this, Zhu et al. have indeed shown that the platelet aggregation results that were not different between vegetarians and omnivores at baseline, became different after 1 and 2 months of supplementation of 450 mg/day choline. Therefore, since the intervention was identical in both groups, the results strongly suggest the presence of effect modifications via yet unknown factors.
Zhu et al. have shown that aspirin lowers plasma TMAO after choline load by almost 50% within 1 month (1). This could be related to changing gastrointestinal acidity and bacterial populations, thus affecting the production rate of TMA; affecting FMO3 system; or affecting a yet unknown TMA-metabolizing system. The results also draw attention to the role of aspirin (and possibly many other drugs) as an effect modifier in clinical studies on the role of TMAO in vascular diseases.
If the study of Zhu et al. (1) is to be used for synthesizing evidence, the following arguments can be made: the hypothesis could be “exposure to TMAO causes thrombosis (shown by using an appropriate surrogate test)” (Figure 1). A randomized controlled trial would be an appropriate design. However, dietary intakes of other sources of TMAO should be controlled and confounding from aspirin or other well-known factors (i.e., renal dysfunction, inflammation, or vascular diseases) that affect TMAO and simultaneously the outcome “platelet aggregation” should be conditioned on. Information on short and long term effects of high choline intake is equally important because of platelet adaptation and analytical limitations of most available surrogate in vitro tests. Since the effect does not appear to further increase over time, resistance or adaptation to high TMAO could be equally a valid explanation.
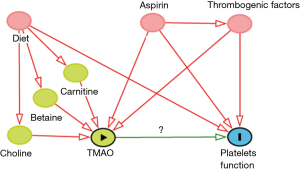
Taken together, because of serious limitations in the study design, inappropriate surrogate outcomes, unknown kinetics of the response of platelets to TMAO, and uncontrolled confounders there is a risk of using such data for causal inference on a proposed direct prothrombotic effect of dietary choline.
Acknowledgements
Funding: None.
Footnote
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2017.08.05). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhu W, Wang Z, Tang WH, et al. Gut Microbe-Generated Trimethylamine N-Oxide From Dietary Choline Is Prothrombotic in Subjects. Circulation 2017;135:1671-1673. [Crossref] [PubMed]
- Kottke-Marchant K, Corcoran G. The laboratory diagnosis of platelet disorders. Arch Pathol Lab Med 2002;126:133-46. [PubMed]
- Paniccia R, Priora R, Liotta AA, et al. Platelet function tests: a comparative review. Vasc Health Risk Manag 2015;11:133-48. [Crossref] [PubMed]
- Gurbel PA, Bliden KP, DiChiara J, et al. Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation 2007;115:3156-64. [Crossref] [PubMed]
- Taylor ML, Misso NL, Stewart GA, et al. The effects of varying doses of aspirin on human platelet activation induced by PAF, collagen and arachidonic acid. Br J Clin Pharmacol 1992;33:25-31. [Crossref] [PubMed]
- Puurunen MK, Hwang SJ, O'Donnell CJ, et al. Platelet function as a risk factor for venous thromboembolism in the Framingham Heart Study. Thromb Res 2017;151:57-62. [Crossref] [PubMed]
- Weber M, Gerdsen F, Gutensohn K, et al. Enhanced platelet aggregation with TRAP-6 and collagen in platelet aggregometry in patients with venous thromboembolism. Thromb Res 2002;107:325-8. [Crossref] [PubMed]
- Li D, Sinclair A, Mann N, et al. The association of diet and thrombotic risk factors in healthy male vegetarians and meat-eaters. Eur J Clin Nutr 1999;53:612-9. [Crossref] [PubMed]
- Li D, Sinclair A, Wilson A, et al. Effect of dietary alpha-linolenic acid on thrombotic risk factors in vegetarian men. Am J Clin Nutr 1999;69:872-82. [PubMed]
Cite this article as: Obeid R. Trimethylamine N-oxide and platelets aggregation: insufficient evidence for causal inference in thrombosis. AME Med J 2017;2:131.

