
Cardiovascular comorbidities in patients with chronic Chagas disease
Introduction
Chagas disease (DC) has as etiological agent the protozoan Trypanosoma cruzi. It is endemic in 21 Latin American countries and is considered one of the 17 neglected tropical diseases in the world in which it affects about 8 to 10 million people, 3 million in Brazil alone. In addition, it is estimated that there are more than 25 million people living in an area of transmission risk (1,2).
For years the main form of transmission of CD was the vector, through contact with the feces of the insect vector of the family Reduviidae, subfamily Triatominae. However, CD control programs in Latin America have caused the Pan American Health Organization (PAHO) to declare some countries free from transmission by the main vector, Triatoma infestans. Thus, other transmission mechanisms support CD permanence such as blood transfusions, organ transplants, accidents with contaminated material, congenital (vertical), consumption of food containing the parasite and other triatomine species (1-3).
The interruption of transmission by the main vector and the efforts to detect CD in blood bank screening significantly reduced acute cases in Brazil (4). On the other hand, individuals infected in the past have evolved into the chronic phase of CD and are currently at an advanced age, which favors the appearance of chronic-degenerative comorbidities that increase cardiovascular risk, hinder their treatment by the health services and affect their quality of life, besides providing the worsening of the CD. Among them, hypertension (HA), diabetes mellitus (DM), dyslipidemia, hypothyroidism, overweight and other heart diseases (5-7).
Thus, the objective of the present study was to describe the clinical-epidemiological profile and to identify the risk factors for cardiovascular diseases of individuals with chronic CD attended at the Clinic for Tropical Diseases of the Medical School of Botucatu (HCFMB), São Paulo, Brazil.
Methods
This is a cross-sectional, descriptive, non-experimental and quantitative study. We included patients with diagnosis of CD in its chronic phase, both sexes, assisted at the Clinic for Tropical Diseases of the Medical School of Botucatu, UNESP during 2014 to 2016.
Data were collected through medical interview, regarding age, gender, place of birth, origin, tobacco use disorder, alcoholism, HA, DM, sedentary lifestyle, dyslipidemia, body mass index (BMI) and clinical form.
To classify the tobacco use, patients were grouped into three categories, according to the World Health Organization (WHO), in which “non-smokers” were those who have never smoked or smoked less than 100 cigarettes during their whole life; “ex-smokers” were those who have smoked at least 100 cigarettes during their life, but stopped; and “current smokers” were those who have smoked 100 or more cigarettes during their lives and are still smoking (8).
Sedentary lifestyle was verified through adaptations of the guidelines of the International Physical Activity Questionnaire (IPAQ). The classification of the physical activity level was divided into three categories: “sedentary” when the patients do not perform any physical activity; “active” when they perform any vigorous physical activity more than three times a week for over 20 minutes and; “very active” when they perform any vigorous physical activity more than five times a week for over 30 minutes (9).
To determine the alcoholism degree, the criteria of the WHO were used: “moderate consumption” for those who drink at least two doses of alcohol a day, or more than twice a week; “social drinking” when they drink alcohol in the company of other people and only for socially acceptable reasons and manners; “no consumption” for those who never have consumed alcohol. Other definitions are not reported since our study did not find them (10).
After obtaining anthropometric data (weight and height), weight was classified according to the BMI through parameters established by the WHO with the formula “weight (kg)/height (m2)”. The patients were grouped as follows: underweight (BMI <18.5 kg/m2), eutrophic (BMI: 18.5–24.9 kg/m2), overweight (BMI: 25.0–29.9 kg/m2), obesity I (BMI: 30.0–34.9 kg/m2), obesity II (BMI: 35.0–39.9 kg/m2) and obesity III (BMI ≥40 kg/m2) (11).
HA, DM and dyslipidemia diagnoses were conducted by a doctor during monitoring of patients, which were classified according to the presence or absence of the pathology by consulting the Electronic Patient Record.
The definitions of clinical manifestations followed the criteria of the II Brazilian Consensus on Chagas Disease: chronic Chagas heart disease (CCHD), the chronic Chagas gastrointestinal disease (CCGD), indeterminate form of human chronic CD (IFCCD) and the mixed form of chronic CD (MFCCD), when there are clinical symptoms of CCHD and CCGD. Those who were diagnosed with CCHD presented changes in the electrocardiography (ECG) and/or echocardiography and clinical symptoms such as palpitations, arrhythmia, ventricular extrasystole, tachycardia and different degrees of heart block. Those diagnosed with CCGD had radiological changes in the esophagus and colon, through barium enema and esophagus, stomach and duodenum (ESD) contrasted study. Patients with IF had no changes in the ECG, barium enema and ESD exams and no clinical symptoms.
The data were entered in the software Microsoft Office Excel 2013 and analyzed through the Epi-Info™ software, version 7.2, provided by the Center for Disease Control and Prevention, which enabled descriptive analysis of frequency and association of variables.
All patients underwent serology for confirmation of CD diagnosis through enzyme-linked immunosorbent assay (ELISA), hemagglutination inhibition test (HAI) and indirect fluorescent antibody technique (IFAT) with IgG research.
Consent to participate in the study was obtained after information and clarification on the research objectives and signature of the informed consent form. The research was submitted to the Research Ethics Committee of the Medical School of Botucatu, UNESP.
Results
In this study, 168 patients with CD in the chronic phase were included, being 85 (50.6%) women and 83 (49.4%) men. The age of patients ranged from 36 to 89 years old, with a 60.8±8.5 years mean, being 60.1±8.2 for women and 61.5±8.8 years for men. The profile of the patients included is present in Table 1. It is worth mentioning that 63.9% of the men were older than 60 years old while the 51.8% women were younger than 60 years old. The clinical manifestations CCHD, CCGD and MFCCD showed percentage of 69.2% in individuals over 60 years old.
Table 1
| Variables | Men (n=83) | Women (n=85) | Total (n=168) | |||||
|---|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | |||
| Age group | ||||||||
| 30 to 39 years old | 0 | 0.00 | 1 | 1.18 | 1 | 0.60 | ||
| 40 to 49 years old | 9 | 10.84 | 5 | 5.88 | 14 | 8.33 | ||
| 50 to 59 years old | 21 | 25.30 | 38 | 44.71 | 59 | 35.12 | ||
| 60 to 69 years old | 39 | 46.99 | 31 | 36.47 | 70 | 41.67 | ||
| 70 to 79 years old | 13 | 15.66 | 9 | 10.59 | 22 | 13.10 | ||
| 80 to 89 years old | 1 | 1.20 | 1 | 1.18 | 2 | 1.19 | ||
| Ethnicity | ||||||||
| White | 62 | 74.70 | 71 | 83.53 | 133 | 79.17 | ||
| Black | 12 | 14.46 | 10 | 11.76 | 22 | 13.10 | ||
| Brown | 9 | 10.84 | 4 | 4.71 | 13 | 7.74 | ||
| Clinical manifestation | ||||||||
| Cardiac | 18 | 21.69 | 15 | 17.65 | 33 | 19.64 | ||
| Digestive | 13 | 15.66 | 22 | 25.88 | 35 | 20.83 | ||
| Mixed | 6 | 7.23 | 4 | 4.71 | 10 | 5.95 | ||
| Indeterminate | 46 | 55.42 | 44 | 51.76 | 90 | 53.57 | ||
| Physical activity | ||||||||
| Very active | 12 | 14.46 | 12 | 14.12 | 24 | 14.29 | ||
| Active | 7 | 8.43 | 10 | 11.76 | 17 | 10.12 | ||
| Sedentary | 64 | 77.11 | 63 | 74.12 | 127 | 75.60 | ||
| Tobacco use disorder | ||||||||
| Smoker | 12 | 14.46 | 12 | 14.12 | 24 | 14.29 | ||
| Former smoker | 43 | 51.81 | 18 | 21.18 | 61 | 36.31 | ||
| Non-smoker | 28 | 33.73 | 55 | 64.71 | 83 | 49.40 | ||
| Alcoholism | ||||||||
| Daily | 2 | 2.41 | 2 | 2.35 | 4 | 2.38 | ||
| Occasionally | 29 | 34.94 | 7 | 8.24 | 36 | 21.43 | ||
| Does not consume | 52 | 62.65 | 76 | 89.41 | 128 | 76.19 | ||
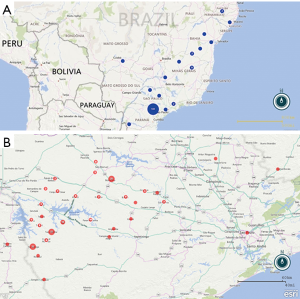
Regarding place of birth, the Figure 1A shows 125 (74.4%) were born in the State of São Paulo, 22 (13.1%) in the state of Paraná, 11 (6.5%) in the state of Minas Gerais and 10 (6.0%) in other states. Among the patients born in the state of São Paulo, 54 (43.2%) belonged to municipalities from Itapeva microregion, including Itaporanga (17.6%), Taquarituba (9.6%) and Coronel Macedo (5.6%). Considering the current hometown, 70 municipalities were mentioned, but three of them outstand: Itaporanga (22–13.1%), Itaí (14–8.3%) and Taquarituba (12–7.1%) (Figure 1B).
The frequency of clinical manifestation was 90.0–53.7% to IFCCD, followed by 35.0–20.8% to CCGD, 33.0–19.6% for CCHD and 10.0–5.9% to MFCCD. There was little difference regarding IFCCD frequency between the sexes; however, for the symptomatic clinical manifestations (CCHD, CCGD and MFCCD) women were affected by the DF (22.0–25.8%) while men were affected by the CCHD (18.0–21.6%). The symptomatic clinical manifestations had frequency in the age group from 60 to 69 years for both sexes, while IF was frequent in the age group from 50 to 59 years.
Considering the BMI, 110 (65.5%) patients were overweight, of which 48 (28.6%) had some degree of obesity. When asked about life habits, 127 (75.6%) do not do any type of physical activity, 24 (14.3%) were smokers and 40 (23.8%) reported consuming alcohol daily or occasionally.
According to Table 2, out of the 168 patients with chronic CD, 86 (51.2%) had HA as comorbidity, 77 (45.8%) had dyslipidemia, 40 (23.8%) had DM, 11 (6.6%) had hypothyroidism and 9 (5.4%) had some type of cardiovascular complication, such as stroke and myocardial infarction. Table 3 shows number of comorbidities of the patients included in the study.
Table 2
| Comorbidities | Cardiac (n=33) | Digestive (n=35) | Mixed (n=10) | Indeterminate (n=90) | Total (n=168) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | % | Number | % | Number | % | Number | % | Number | % | |||||
| BMI (kg/m2) | ||||||||||||||
| BMI ≤18.5 (underweight) | 0 | 0.00 | 1 | 2.86 | 0 | 0.00 | 1 | 1.11 | 2 | 1.19 | ||||
| BMI >18.5 and ≤24.9 (eutrophic) | 12 | 36.36 | 11 | 31.43 | 6 | 60.00 | 27 | 30.00 | 56 | 33.33 | ||||
| BMI ≥25.0 and ≤29.9 (overweight) | 10 | 30.30 | 7 | 20.00 | 2 | 20.00 | 43 | 47.78 | 62 | 36.90 | ||||
| BMI ≥30.0 and ≤34.9 (obesity I) | 7 | 21.21 | 11 | 31.43 | 2 | 20.00 | 14 | 15.56 | 34 | 20.24 | ||||
| BMI ≥35.0 and ≤39.9 (obesity II) | 4 | 12.12 | 4 | 11.43 | 0 | 0.00 | 3 | 3.33 | 11 | 6.55 | ||||
| BMI ≥40.0 (obesity III) | 0 | 0.00 | 1 | 2.86 | 0 | 0.00 | 2 | 2.22 | 3 | 1.79 | ||||
| Hypertension | ||||||||||||||
| Yes | 24 | 72.73 | 18 | 51.43 | 6 | 60.00 | 38 | 42.22 | 86 | 51.19 | ||||
| No | 9 | 27.27 | 17 | 48.57 | 4 | 40.00 | 52 | 57.78 | 82 | 48.81 | ||||
| Diabetes | ||||||||||||||
| Yes | 11 | 33.33 | 6 | 17.14 | 1 | 10.00 | 22 | 24.44 | 40 | 23.81 | ||||
| No | 22 | 66.67 | 29 | 82.86 | 9 | 90.00 | 68 | 75.56 | 128 | 76.19 | ||||
| Dyslipidemia | ||||||||||||||
| Yes | 20 | 60.61 | 19 | 54.29 | 5 | 50.00 | 33 | 36.67 | 77 | 45.83 | ||||
| No | 13 | 39.39 | 16 | 45.71 | 5 | 50.00 | 57 | 63.33 | 91 | 54.17 | ||||
| Hypothyroidism | ||||||||||||||
| Yes | 4 | 12.12 | 2 | 5.71 | 0 | 0.00 | 5 | 5.56 | 11 | 6.55 | ||||
| No | 29 | 87.88 | 33 | 94.29 | 10 | 100.00 | 85 | 94.44 | 157 | 93.45 | ||||
| Cardiovascular complications | ||||||||||||||
| Yes | 1 | 3.03 | 3 | 8.57 | 1 | 10.00 | 4 | 4.44 | 9 | 5.36 | ||||
| No | 32 | 96.97 | 32 | 91.43 | 9 | 90.00 | 86 | 95.56 | 159 | 94.64 | ||||
Table 3
| Number of comorbidities | Distribution | % |
|---|---|---|
| 4 comorbidities | 7 | 4.2 |
| 3 comorbidities | 14 | 8.3 |
| 2 comorbidities | 46 | 27.4 |
| 1 comorbidity | 61 | 36.3 |
| No comorbidity | 40 | 23.8 |
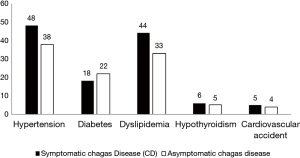
It is worth mentioning that, as shown in Figure 2, HA, dyslipidemia, hypothyroidism and cardiovascular complications were observed with regular times frequent in patients who had a defined clinical manifestation, except DM. Hence, it is worth highlighting HA and dyslipidemia as frequent comorbidities associated with chronic CD, with 55.8% and 57.1% respectively. The comorbidities such as HA was observed in 40.1% of patients and dyslipidemia was observed in 33.6% and the age range of this group of patients were over 60 years.
Discussion
As aforementioned, CD is a public health problem with many aspects not yet clarified, particularly the cardiovascular risk factors related to the disease. This study observed no significant difference between the sexes, although most men included in the study were diagnosed in blood banks after the donation. Thus, our findings are consistent with data found in the literature, according to which the CD affects men and women indistinctly. On the other hand, some authors suggest that the male prevalence in the CD is significantly lower comparing with women, since women seek health services more often for routine exams and prevention than men; man’s diagnosis is fair and less notify than adults woman (12,13).
Regarding age, both sex and the age group found (60.8±8.5 years) were lower than those found by de Almeida et al. (2007) and Alves et al. (2009), although still within the age group considered young elderly. The mean age of younger individuals (under 60 years) was 53.4±5.6 years, which could mean aging of individuals infected in that age group. Therefore, aging of this population can favor the emergence of comorbidities that affect the quality of life of patients, which can worsen the prognosis if associated with this disease. The association of chronic CD with comorbidities such as HA, DM and dyslipidemia represent a major challenge to public health (7,13,14).
Regarding clinical manifestations of chronic CD, there was predominance (53.5%) of IFCCD for both sexes, which is in agreement with other authors. This may be related to the fact that the study was conducted in patients of an outpatient clinic. When compared with the age group, IFCCD was more frequent (67.5%) in patients under 60 years old, while the symptomatic clinical manifestations were more frequent in individuals over 60 years old (69.2%). Although asymptomatic, presence of parasites may trigger inflammatory responses, which contributes to the emergence and aggravation of cardiovascular changes. These changes and other comorbidities can reduce time and quality of life of infected individuals (5,15-17).
The symptomatic clinical manifestations (CCHD, CCGD and MFCCD) are manifestations of the chronic phase of CD. They may develop late in approximately 30% to 40% of the infected individuals. Among them, CCHD is the most severe manifestation of the disease, with complex physiopathology and big impact on indicators of morbidity and mortality. Our findings are consonant with many authors, who relate the CCHD to men. In this study, CCHD was reported in 21.7% of men and 17.6% of women (6,7,18,19).
CCGD affects approximately 10% of individuals with chronic CD, with digestive manifestations as megacolon and esophageal achalasia. Clinical signs vary from asymptomatic motility disorders to more severe sings such as deglutition disorders, gastroesophageal reflux, chronic constipation and weight loss (20).
When it comes to treating the individual with chronic CD with little active and potentially toxic drugs, the objective must always focus on controlling the symptoms and improving quality of life. Thus, the treatment of comorbidities that affect them can prevent future complications as well as limit the progression of the disease and increase the survival rate of these individuals. The comorbidities evaluated in the study—obesity, HA, DM, dyslipidemia, hypothyroidism, cardiovascular complications, tobacco use disorder, alcoholism and sedentary lifestyle—in addition to mortality, can cause irreversible damage and directly affect the quality of life of individuals and, if they happen simultaneously, there may be an increased risk comparing with them separately (21,22).
In Brazil, HA affects approximately 32.5% of the adult population and is directly or indirectly responsible for 50% of deaths caused by cardiovascular diseases. In this study, HA was the most reported comorbidity (51.2%) among individuals with chronic CD, the same found in several studies, such as Silva et al. (2010), who found HA in 64% of the individuals studied. According to the Brazilian Society of Cardiology (2016), about 60% of the hypertensive subjects are older adults older than 60 years, similar to the number we found, which was 63.9%. These data suggest that CD may be responsible for blood pressure elevation, especially if it comes along with aging of the individuals (13,23-25).
By evaluating all the clinical manifestations, we noticed that HA was more frequent in IFCCD; however, when the clinical manifestations were individually analyzed, the prevalence was in individuals with CCHD. This prevalence was also found by Bertanha et al. (2008) and can be justified by the advanced age of patients with CCHD and more time for the disease evolution. Thus, the incidence of HA with the chronic CD is a factor that must be considered in the follow-up of the individual, since this situation can result in a functional impairment of the cardiovascular system (26,27).
Dyslipidemia was the second comorbidity that most affected individuals with chronic CD, which was found in 41.6% of them, higher than the number found by Alves et al. (2009) in a study with individuals with chronic CD at the Clinic Hospital of the University of Campinas. Dyslipidemia along with HA represent important factors of initiation and development of cardiovascular diseases. Another study by Teston et al. (2016), found over 50% individuals with changes in lipid levels, with odds 2.6 higher of developing a cardiovascular disease than normal individuals (13,28,29).
In this study, DM frequency was low. There are very few studies that relate DM with chronic CD; however, Campos and Cançado (1962) showed that patients with the IFCCD showed lower insulin response. Oliveira et al. (1993) demonstrated parasympathetic denervation and injury in the pancreatic cells. Among the individuals with DM, 75% presented overweight and obesity. This association between excess of weight and DM becomes even more important in the Brazilian population, since according to studies, over half of people are overweight. Therefore, it is suggested that DM is a comorbidity related to modifiable risk factors, and when combined with other comorbidities, it raises the risk of cardiovascular diseases (30-33).
Another important aspect in this study is associated with excess of weight. This finding may contribute due to the prevalence of HA and dyslipidemia as observed. In this study, over 65% of the individuals were overweight and obese. This demonstrates the importance of adopting strategies aimed at encouraging healthy eating habits and physical activity to control several risk factors for cardiovascular diseases.
Among life habits, tobacco use disorder and alcoholism were not significant (14.3% and 23.8%, respectively). However, sedentary lifestyle was observed in 75.6% of individuals. The evaluation of sedentary lifestyle is very important in the development of cardiovascular diseases. Every year, approximately 3.2 million deaths are caused by insufficient physical activity, a factor already considered as public health priority in the world. According to data from the Brazilian Institute of Geography and Statistics (IBGE), in Brazil, sedentary lifestyle reaches about 80.8% of adults. Only in São Paulo city, Mello et al. (2000) verified a 68.7% rate of sedentary adults (34). In a study conducted in Bambuí, in the state of Minas Gerais, the sedentary lifestyle was observed in 29.8% out of the 1,479 patients with the CD (>60 years old). Similar results to ours were found by Geraix et al. (2007), in a retrospective data collection with CD patients assisted at the same outpatient clinic of our study, which reported that 83.3% were sedentary. It shows that in our study population, many individuals are sedentary. This is really worrying, since the sedentary lifestyle can control cognitive functions negatively, increase the risk of myocardial infarction, as well as be a risk factor for many chronic diseases (17,35-38).
Regarding hypothyroidism, a low frequency was observed in the individuals studied. Hypothyroidism is one of the most common endocrine disorders and is characterized by a deficiency in the production of the thyroid hormone. Its prevalence varies, but it is known that individuals at an advanced age and women are the most affected. Although the symptoms are not specific, we know that hypothyroidism is associated with weight gain, HA and cardiovascular changes, which constitute important comorbidities in the chronic CD (39-41)
There was no association between low frequency of cardiovascular complications and other comorbidities as well as the clinical manifestation of the chronic CD.
Conclusions
Finally, we can conclude that most individuals with chronic CD identified in the outpatient service are older adults, a particularly vulnerable population both for the CD and the association of comorbidities. These comorbidities, individual or associated, may aggravate the health condition of the individual and cause serious cardiovascular damage. In the absence of a specific treatment for the chronic CD, it is important to adopt measures aimed at the control of these comorbidities and that favor the quality of life of these individuals.
Acknowledgements
The authors thank the nursing team and physicians of the Ambulatory of Tropical Diseases of the Clinical Hospital of the Botucatu Medical School and the Graduate Program in Tropical Diseases of Botucatu Medical School (UNESP).
Funding: This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES) masters scholarship for the student Luiz Roberto de Oliveira Junior and Thaysa Buss Carvalho.
Footnote
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.07.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Consent to participate in the study was obtained after information and clarification on the research objectives and signature of the informed consent form. The research was submitted to the Research Ethics Committee of the Medical School of Botucatu, UNESP (No. 1.576.519/2016).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brasil. Guia de Vigilância em Saúde. 1a Edição. Brasília, 2014. 812 p.
- Martins-Melo FR, Ramos AN Jr, Alencar CH, et al. Prevalence of Chagas disease in Brazil: a systematic review and meta-analysis. Acta Trop 2014;130:167-74. [Crossref] [PubMed]
- Rassi A Jr, Rassi A, Marcondes de Rezende J. American Trypanosomiasis (Chagas Disease). Infect Dis Clin North Am 2012;26:275-91. [Crossref] [PubMed]
- Lee BY, Bacon KM, Bottazzi ME, et al. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect Dis 2013;13:342-8. [Crossref] [PubMed]
- Junqueira LF Jr. Insights into the clinical and functional significance of cardiac autonomic dysfunction in Chagas disease. Rev Soc Bras Med Trop 2012;45:243-52. [Crossref] [PubMed]
- Guariento ME, Alliegro FC, De Almeida EA, et al. Chagas’ disease associated with chronic infirmities in outpatients followed in a university hospital. Rev Bras Clin Med 2009;7:84-8.
- de Almeida EA, Barbosa Neto RM, Guariento ME, et al. Clinical presentation of chronic Chagas disease in elderly individuals. Rev Soc Bras Med Trop 2007;40:311-5. [PubMed]
- WHO. Guidelines for controlling and monitoring the tobacco epidemic. World Health Organization, 1998.
- Matsudo S, Araújo T, Matsudo V, et al. Questionário Internacional De Atividade Física (Ipaq): Estupo De Validade E Reprodutibilidade No Brasil. Rev Bras Atividade Física Saúde 2012;6:5-18.
- WHO | Lexicon of alcohol and drug terms published by the World Health Organization. WHO 2010 [cited 2017 Apr 18]. Available online: http://www.who.int/substance_abuse/terminology/who_lexicon/en/#.WPX-DmsRJ98.mendeley&title=WHO %7C Lexicon of alcohol and drug terms published by the World Health Organization
- WHO. Obesity: preventing and managing the global epidemic. World Health Organization, 2000.
- Gomes R, Nascimento EF, do , de Araújo FC. Por que os homens buscam menos os serviços de saúde do que as mulheres? As explicações de homens com baixa escolaridade e homens com ensino superior. Cad Saude Publica 2007;23:565-74. [Crossref] [PubMed]
- Alves RM, Thomaz RP, Almeida EA, et al. Chagas' disease and ageing: the coexistence of other chronic diseases with Chagas' disease in elderly patients. Rev Soc Bras Med Trop 2009;42:622-8. [Crossref] [PubMed]
- Guariento ME, Carrijo CM, de Almeida EA, et al. Perfil clínico de idosos portadores de doença de Chagas atendidos em serviço de referência. Rev Bras Clin Med 2011;9:20-4.
- Navarro EC, de Abreu MM, Tavares FC, et al. Indeterminate Form of Chagas’ Disease and Metabolic Syndrome: A Dangerous Combination. Am J Med Med Sci 2013;3:68-73.
- Bozelli CE, de Araújo SM, Guilherme AL, et al. Perfil clínico-epidemiológico de pacientes com doença de Chagas no Hospital Universitário de Maringá, Paraná, Brasil. Cad Saude Publica 2006;22:1027-34. [Crossref] [PubMed]
- Geraix J, Ardisson LP, Marcondes-Machado J, et al. Clinical and nutritional profile of individuals with Chagas disease. Braz J Infect Dis 2007;11:411-4. [Crossref] [PubMed]
- Borges-Pereira J, Zauza PL, Galhardo MC, et al. Doença de Chagas na população urbana do distrito sanitário de Rio Verde, Mato Grosso do Sul, Brasil. Rev Soc Bras Med Trop 2001;34:459-66. [Crossref] [PubMed]
- Assunção AN Jr, Jerosch-Herold M, Melo RL, et al. Chagas' heart disease: gender differences in myocardial damage assessed by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2016;18:88. [Crossref] [PubMed]
- Bonney KM. Chagas disease in the 21st century: a public health success or an emerging threat? Parasite 2014;21:11. [Crossref] [PubMed]
- Munari DB, Lucchese R, Medeiros M. Reflexões sobre o uso de atividades grupais na atenção a portadores de doenças crônicas. Ciência Cuid e Saúde 2009;8:148-54.
- Oliveira BG, Abreu MN, Abreu CD, et al. Health-related quality of life in patients with Chagas disease. Rev Soc Bras Med Trop 2011;44:150-6. [Crossref] [PubMed]
- Silva EM, Rocha MO, Silva RC, et al. Clinic and epidemiological study on Chagas disease in the Serra Azul district of Mateus Leme, central-western region of the State of Minas Gerais, Brazil. Rev Soc Bras Med Trop 2010;43:178-81. [Crossref] [PubMed]
- Sociedade Brasileira de Cardiologia. 7a Diretriz Brasileira de Hipertensão Arterial. Rio de Janeiro (RJ) 2016;107. 104 p.
- Gurgel CB, Miguel Junior A, Mendes CR, et al. Frequency of hypertension in chronic Chagas' disease: retrospective clinical study. Arq Bras Cardiol 2003;81:545-8, 541-4.
- Bertanha L, Guariento ME, Magna LA, et al. Caracterização clínico-laboratorial de chagásicos hipertensos sem insuficiência cardíaca manifesta. Rev Soc Bras Med Trop 2008;41:163-8. [Crossref] [PubMed]
- Souza LRMF, Guariento ME. Evolução de pacientes chagásicos acompanhados em um serviço de referência. Rev Soc Bras Med Trop 1998;31:54-5.
- Moreira OC, de Oliveira CEP, Teodoro BG, et al. Fatores de risco de doença cardiovascular em técnicos administrativos da Universidade Federal de Viçosa. Biosci J 2009;25:133-40.
- Teston EF, Cecilio HPM, Santos AL, et al. Factors associated with cardiovascular diseases in adults. Med (Ribeirao Preto Online) 2016;49:95. Available online: http://www.journals.usp.br/rmrp/article/view/118390/115943
- Siqueira AF. Almeida-Pititto Bd, Ferreira SR. Cardiovascular disease in diabetes mellitus: classical and non-classical risk factors. Arq Bras Endocrinol Metabol 2007;51:257-67. [Crossref] [PubMed]
- Oliveira LC, Juliano Y, Novo NF, et al. Blood glucose and insulin response to intravenous glucose by patients with chronic Chagas' disease and alcoholism. Braz J Med Biol Res 1993;26:1187-90. [PubMed]
- Flor LS, Campos MR, Oliveira AF, et al. Diabetes burden in Brazil: fraction attributable to overweight, obesity, and excess weight. Rev Saude Publica 2015;49:29. [Crossref] [PubMed]
- Campos JO, Cançado JR. Curvas glicêmicas anormais observadas em pacientes com a forma crônica da moléstia da Chagas. Hospital (Rio de J) 1962;62:275-8.
- Mello MT, Fernandez AC, Tufik S. Levantamento epidemiológico da prática de atividade física na cidade de São Paulo. Rev Bras Med Esporte [online]. 2000;6:119-24. Available online: http://dx.doi.org/
10.1590/S1517-86922000000400003 - IBGE. Pesquisa Nacional de Saúde [Internet]. Rio de Janeiro (RJ); 2014. 181 p. [cited 2017 Jul 4]. Available online: ftp://ftp.ibge.gov.br/PNS/2013/pns2013.pdf
- Volkers KM, Scherder EJ. Impoverished environment, cognition, aging and dementia. Rev Neurosci 2011;22:259-66. [Crossref] [PubMed]
- Gawryszewski VP, Koizumi MS, de Mello-Jorge MHP. As causas externas no Brasil no ano 2000: comparando a mortalidade e a morbidade. Cad Saude Publica 2004;20(4):995-1003. [cited 2017 Mar 30] Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-311X2004000400014&lng=pt&nrm=iso&tlng=pt
- WHO. WHO | Physical Inactivity: A Global Public Health Problem [Internet]. WHO. World Health Organization; 2014 [cited 2017 Jul 4]. Available online: http://www.who.int/dietphysicalactivity/factsheet_inactivity/en/
- Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med 2001;344:501-9. [Crossref] [PubMed]
- Carlé A, Pedersen IB, Knudsen N, et al. Gender differences in symptoms of hypothyroidism: a population-based DanThyr study. Clin Endocrinol (Oxf) 2015;83:717-25. [Crossref] [PubMed]
- Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull 2011;99:39-51. [Crossref] [PubMed]
Cite this article as: Oliveira Junior LR, Carvalho TB, da Costa ÉAPN, Pereira PCM, Kurokawa CS. Cardiovascular comorbidities in patients with chronic Chagas disease. AME Med J 2018;3:79.

