
Thrombophilia testing in mesenteric venous thrombosis, when to screen
Clarification of nomenclature
Al-Samkari et al. present an approach to thrombophilia testing in patients with splanchnic vein thrombosis (SVT) (1). They suggest an algorithm highlighting the importance to screen for local precipitating factors and any blood count disturbances before testing for thrombophilia including Janus kinase 2 v617F mutation (JAK2) (1). Authors state that the majority of these local precipitating factors will be diagnosed or excluded on the computed tomography (CT) or magnetic resonance imaging (MRI), modalities frequently used for diagnosis of SVT in the vast majority of cases (1,2).
Recognition is important for a disease that is under-appreciated, but when comparing different strategies, we must bear in mind that the commonly used nomenclature is confusing. The topic of the review by Zarrouk et al. (2) was mesenteric venous thrombosis (MVT), not SVT, a term often incorporating thrombosis of the hepatic veins as well. MVT and venous mesenteric ischaemia are synonymous terms, emphasizing that this disease entity is an acute abdominal condition with a high risk of developing intestinal infarction and death. MVT should not be confused with isolated portal vein thrombosis in patients with liver cirrhosis or with the Budd-Chiari syndrome (3). Due to its emergency, MVT is not a condition often diagnosed by magnetic resonance imaging (MRI). Unfortunately, it is evident from the commentary by Al-Samkari that we do not always discuss the same disease entity outlined in the original systematic review (2). Diagnosis with MRI and risk factor evaluation with ultrasound of the heart and liver pertains to a more chronic disease entity, perhaps more confined to the liver, and are not always relevant in MVT.
Disparities in risk factor evaluation
As already stated by Zarrouk et al. (2), there is a well-known association with Virchow’s triad in the development of MVT, therefore, we do agree that local precipitating factors must be evaluated by CT scanning exclusively for any patient with MVT (2). According to the algorithm proposed by Al-Samkari et al., however, both heart failure and portal hypertension should be excluded before considering thrombophilia testing, a strategy requiring ultrasound (US) of both the heart and the liver (1). At our institution, Skåne University Hospital, the price for the former is 2800 SEK (272 €; www.oanda.com; accessed 01 Jun 2018) and for the latter 1139 SEK (111 €). In comparison, testing for thrombophilia including inherited thrombophilia factors [factor V Leiden (FVL) mutation, prothrombin (PT) gene mutation and deficiencies of protein C (PC), protein S (PS), antithrombin (AT)] and acquired thrombophilia factors [JAK2, lupus anticoagulant (LA) and cardiolipin antibodies] costs 3116 SEK (302 €) (4).
Zarrouk et al. showed that prevalences of heart failure [2/107 (2%)] and portal hypertension [14/120 (12%)] were both lower than that of any inherited or acquired thrombophilia factor (35% or 14%, respectively) (2). Interestingly, the use of the N-terminal prohormone of brain natriuretic peptide (NT-proBNP), a well-known biomarker for the diagnosis of heart failure (5), incurs a much lower cost 169 SEK (144 €) compared to US (4,6). Screening all MVT patients with US of the heart and liver would be an expensive strategy yielding few diagnostic findings.
The extension of thrombosis within the portomesenteric venous system
Importantly, Al-Samkari et al. highlight the importance to separate SVT into four subtypes (mesenteric, portal, splenic, and multiple veins) due to different associations with the various thrombophilias. In addition to this, we also know that outcomes, such as mortality, varies between the above mentioned subtypes (7). It should be clear that thrombosis within the superior mesenteric vein carries a high risk of intestinal infarction and relatively high short-term mortality in comparison with isolated portal vein thrombosis (7).
Management of MVT
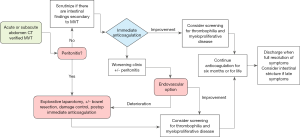
Importantly, in the acute phase of the disease, the initiation of treatment with low molecular weight heparin (LMWH) and awareness of complications, such as bowel ischemia and risk of bleeding, should be the clinical priority and not the precise anatomic venous involvement (8,9). Therefore, we added an algorithm illustrating a proposal to the initial evaluation of MVT patients (Figure 1).
Duration of anticoagulation therapy
MVT patients without reversible provocative factors (direct injury, local venous congestion/stasis, hormone therapy, or malignancy), the vast majority of patients will receive indefinite anticoagulation treatment due to their high MVT related mortality (3,10). The principle of indefinite treatment is in line with current American College of Chest Physicians guidelines for venous thromboembolism (VTE), recommending patients “with a first VTE that is an unprovoked proximal deep venous thrombosis (DVT) of the leg or pulmonary embolism (PE) and who have a low or moderate bleeding risk” to receive indefinite anticoagulation treatment (11). For patients in whom the decision of indefinite anticoagulation is made, further thrombophilia testing has no clinical consequences. Importantly, if a reversible provocative factor is present, a time limited period of anticoagulation is possible, given the provoking factor is removed.
Controversies in thrombophilia testing
On the other hand, duration of anticoagulation therapy in patients with an identified non-reversible provoking factor such as FVL mutation is a matter of debate. In a population-based study including 900 VTE patients, patients with heterozygous FVL mutation had an increased risk (odds ratio 2.4; 95% confidence interval: 1.6–3.6) for new VTE recurrence during a mean follow up time of 5 years (12). The high rate of genetic and acquired prothrombotic factors present in patients with MVT (2) and potential severe clinical consequences of recurrence makes experts tend to offer patients with identified laboratory-confirmed thrombophilia indefinite anticoagulation treatment, despite insufficient evidence for such treatment. Consequently, routine laboratory screening may be considered in patients with MVT without an identified provocative factor on CT scan. The European Society of Vascular Surgery guidelines recommend lifelong anticoagulation in patients with MVT with proven thrombophilia (3).
Whom to test for thrombophilia
Finally, it’s important to emphasize that the health care system in Sweden is largely tax-funded, ensuring that everyone has equal access to health care services, free of charge. In the present digital era, patients and relatives have easy access to information concerning diseases. Furthermore, it is not unusual that patients or relatives demand thorough investigation, including thrombophilia testing, to be sure the medical team does everything possible to find their answers and concerns. This fact further emphasizes the importance of reaching professional consensus on whom to screen for thrombophilia. Until further, we agree with the algorithm suggested by Connors on VTE at unusual sites such as MVT (13), advocating testing for inherited thrombophilias and myeloproliferative neoplasms when there are no strong trigger factors present.
Acknowledgements
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal. The article did not undergo external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/amj.2018.07.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Al-Samkari H, Connors JM. Approach to thrombophilia testing in patients with splanchnic vein thrombosis. AME Med J 2018;3:7. [Crossref]
- Zarrouk M, Salim S, Elf J, et al. Testing for thrombophilia in mesenteric venous thrombosis - Retrospective original study and systematic review. Best Pract Res Clin Gastroenterol 2017;31:39-48. [Crossref] [PubMed]
- Björck M, Koelemay M. Management of the diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg 2017;53:460-510. [Crossref] [PubMed]
- Pris 2018 - Region Skåne samt landstingen Blekinge, Södra Halland, Kronoberg. Available online: https://vardgivare.skane.se/siteassets/2.-patientadministration/avgifter-och-prislistor/prislistor/labmedicin/rs---fillistning/klinisk-kemi-2018-rs-v2.pdf
- Groenning BA, Nilsson JC, Sondergaard L, et al. Evaluation of impaired left ventricular ejection fraction and increased dimensions by multiple neurohumoral plasma concentrations. Eur J Heart Fail 2001;3:699-708. [Crossref] [PubMed]
- Bild- och funktionsmedicin prislista 2018 Region Skåne. Available online: https://vardgivare.skane.se/siteassets/2.-patientadministration/avgifter-och-prislistor/prislistor/bfm/prislista-2018-20180514.xlsx
- Acosta S, Alhadad A, Verbaan H, et al. The clinical importance in differentiating portal from mesenteric venous thrombosis. Int Angiol 2011;30:71-8. [PubMed]
- Salim S, Zarrouk M, Elf J, et al. Improved Prognosis and Low Failure Rate with Anticoagulation as First-Line Therapy in Mesenteric Venous Thrombosis. World J Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Salim S, Ekberg O, Elf J, et al. Evaluation of direct oral anticoagulants and vitamin K antagonists in mesenteric venous thrombosis. Phlebology 2018;268355518779517 [Epub ahead of print]. [PubMed]
- Acosta S, Alhadad A, Svensson P, et al. Epidemiology, risk and prognostic factors in mesenteric venous thrombosis. Br J Surg 2008;95:1245-51. [Crossref] [PubMed]
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315-52. [Crossref] [PubMed]
- Sveinsdottir S, Saemundsson Y, Isma N, et al. Evaluation of recurrent venous thromboembolism in patients with Factor V Leiden mutation in heterozygous form. Thromb Res 2012;130:467-71. [Crossref] [PubMed]
- Connors JM. Thrombophilia testing and venous thrombosis. N Engl J Med 2017;377:1177-87. [Crossref] [PubMed]
Cite this article as: Zarrouk M, Salim S, Elf J, Gottsäter A, Acosta S. Thrombophilia testing in mesenteric venous thrombosis, when to screen. AME Med J 2018;3:90.

