Minimally invasive direct coronary artery bypass (MIDCAB) grafting
Introduction
A significant stenosis in the proximal left anterior descending (LAD) artery can jeopardise a large area of the myocardium (1). A variety of therapeutic options, including full-sternotomy on- or off-pump coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI) and minimally invasive direct coronary artery bypass (MIDCAB) grafting, are available to tackle this potentially life-threatening lesion of the LAD. On-pump CABG has become the standard of care for surgical myocardial revascularization world-wide. However, the morbidity associated with cardiopulmonary bypass (CPB) coupled with full-sternotomy approach make on-pump CABG a very invasive strategy for revascularization of isolated LAD. This is an important consideration particularly for high-risk patients. On the other hand, although off-pump CABG through full sternotomy for isolated LAD disease abolishes or at least reduces CPB-associated morbidity (2) yet the potential risk of infective complications associated with full sternotomy persists. MIDCAB avoids CPB and a full sternotomy and thereby eliminates the complications associated with both CPB use and full sternotomy. Most importantly it offers the well-recognised benefits of a left internal mammary artery (LIMA) to LAD that remains the gold standard strategy for revascularization of the LAD and has stood the test of time.
History
The earlier attempts to revascularize the myocardium date back to the middle of the 20th century, a long time before the utilization of CPB for CABG. In 1952, Arthur Vineburg described his technique of myocardial revascularization by directly implanting internal mammary artery into heart muscle as a strategy to improve blood flow to heart muscle (3).
However, it was not until the early 1960s that direct coronary artery grafting was established as the standard in coronary surgery due to the foresight and perseverance of a few surgeons. In 1961, Goetz and associates reported their experience with nonsutured IMA anastomoses. They connected the right IMA (RIMA) to the right coronary artery (RCA) on the beating heart by means of a tantalum ring (4). However, Vasilii I. Kolesov, described as the pioneer and founder of coronary revascularization by Olearchyk (5), deserves the credit for development of minimally invasive coronary surgery at his clinic in St. Petersburg. He introduced direct sutured anastomosis between the IMAs and coronary arteries and performed the first operation in February 1964 (6).
In 1973, Garrett and colleagues reported a case of saphenous vein graft performed on beating heart 9 years earlier in 1964 (7). Trapp and Bisarya in Canada (8) and Ankeney in the United States (9) tried to propagate off-pump CABG against the popular trend of performing CABG with CPB on an arrested heart in a bloodless, motionless surgical field. It took nearly two decades for the perception regarding off-pump CABG to change. In 1995 Benetti proposed the concept of performing CABG in a minimally invasive manner without CPB and through a small incision (10). This innovative approach, which combined the off-pump technique with the minimally invasive approach, came to be known as MIDCAB or the left anterior small thoracotomy (LAST) operation and was further popularized by Calafiore and colleagues (11) and Subramanian and associates (12). It is now widely used by some centres for revascularization of the isolated stenosis of the proximal LAD.
Indications
MIDCAB is indicated for isolated significant proximal LAD disease when PCI is not advisable (complex lesions), not successful, or not possible (occluded LAD). It is also offered to patients who have previously undergone PCI of the LAD and experience return of symptoms due to progression of in-stent stenosis. Similarly, patients with two-vessel disease who have already undergone stenting of a non-LAD culprit vessel as a primary angioplasty procedure can subsequently have MIDCAB grafting of the LAD.
MIDCAB can also be offered as a part of hybrid revascularization in cases where significant proximal LAD stenosis is accompanied by lesions in right and circumflex coronary arteries that are deemed suitable for PCI.
Patients suffering from multiple comorbidities like chronic obstructive pulmonary disease (COPD), chronic renal insufficiency, diffuse cerebrovascular and peripheral vascular disease, malignancy and those with advanced age are at an extremely high risk for CPB-related morbidity. In addition, patients with severe uncontrolled insulin-dependent diabetes mellitus, obesity, renal failure or immune deficiency have a higher predilection for deep sternal wound infections. Such patients would be better served by a MIDCAB procedure followed by PCI to the other vessels if required. Patients with multivessel disease with poor left ventricular function, ischemic cardiomyopathy and congestive heart failure, who are not transplant candidates, have a very high predicted mortality for conventional CABG. Jacobs et al. reported an actuarial 4-year survival of 85.6% and the event-free survival including freedom from angina, MACE, and reintervention of 81.5% in this patient group. They showed that MIDCAB, in comparison to conventional CABG in high-risk patients, carries a lower incidence of in-hospital death, neurological events, and perioperative myocardial infarction with similar midterm result (13).
Patients needing isolated redo CABG due to failure of saphenous vein graft to the LAD can also be offered LIMA to LAD using the MIDCAB approach if PCI is deemed technically impossible.
There are a number of favourable anatomical features listed in Table 1 that facilitate safe and comfortable conduct of the operation and must be minutely scrutinized in every patient prior to undertaking the procedure.
Table 1
| Patient-related |
| Slim patient |
| Thin, tubular, vertically positioned heart |
| LAD-related |
| Non-calcified mid-distal segment (2–4 cm distal to the second diagonal branch) |
| Arterial diameter greater than 1.75 mm in diameter |
| Total occlusion of the LAD with good collaterals to the distal segment |
MIDCAB, minimally invasive direct coronary artery bypass; LAD, left anterior descending artery.
Contraindications
At present, an occluded left subclavian artery and a patient in cardiogenic shock with LAD as the culprit vessel requiring emergent revascularization are the only absolute contraindications to MIDCAB grafting. In the second scenario, as the sole aim is to restore blood flow in the native vessel as quickly and safely as possible, MIDCAB will be a less attractive option due to due to longer time needed to harvest the LIMA and perform the anastomosis. Performance of this operation in an emergency situation like iatrogenic dissection of the LAD in the catheterization laboratory is also controversial. One can argue in favour of a MIDCAB procedure in such a situation when the patient is hemodynamically stable, without overt signs of ongoing ischemia, and the operation is to be performed by an experienced surgeon who can expedite the procedure efficiently. However, for all practical purposes such a scenario should be regarded as a relative contraindication for MIDCAB grafting.
The other relative contraindications for MIDCAB are predominantly dependent on the experience and expertise of the operating surgeon. Presence of a deep intramyocardial, calcified LAD with a diameter less than 1.5 mm poses a real challenge for the operating surgeon. Similarly, previous thoracotomy and extensive chest adhesions are relative contraindications as they restrict the exposure and thus limit the advantage of a minimally invasive approach. The threshold to convert to a sternotomy should be low in such patients.
Extreme obesity makes the operation technically more demanding at every stage. LIMA harvest becomes an arduous task not only due to the difficult exposure through the thick chest wall, but also due to the fact that it is enveloped in a thick layer of fat, especially in its proximal part, making it difficult to visualize. In such patients, the epicardium is laden with fat and exposure of a deeply seated LAD can be technically demanding through a small incision. Finally, performing the LIMA-LAD anastomosis is also more challenging than usual as it has to be performed deep in the chest through the limited access.
Technical aspects
Preoperative evaluation
Preoperative evaluation of the patient is extremely important to ensure that MIDCAB is appropriately offered to the right patient for the correct indication. MIDCAB is best avoided in patients requiring emergent revascularization and those with severe COPD. Thorough physical examination of the patient prior to undertaking MIDCAB is mandatory. Presence of obesity, chest contour (better visualization of the LIMA with increasing curvature of the chest wall) and length of the thorax (proximal LIMA access becomes more difficult with increasing length of the chest) are some of the aspects that must be carefully assessed. The chest radiograph not only validates the physical findings but also enables the surgeon to estimate the position of the heart (horizontal or vertical), its size and the width of the intercostal spaces. The preoperative coronary angiogram is the most important investigation, which allows the surgeon to assess the LAD and determine the technical feasibility of the procedure (Table 2).
Table 2
| LAD course (whether it reaches the apex or falls significantly short of it) |
| LAD depth (epicardial or deep intramyocardial) |
| LAD size (preferably greater than 1.75 mm) |
| LAD wall quality (diffuse disease or calcification) |
| LAD stenosis (higher the grade of stenosis, better is the tolerance to occlusion) |
| Location of accompanying arteries (a large diagonal running parallel to the LAD may be mistaken to be the LAD and grafted) |
| Position of stents if the artery has been previously stented |
MIDCAB, minimally invasive direct coronary artery bypass; LAD, left anterior descending artery.
Patient positioning and monitoring
The patient is placed in a supine position with the left chest elevated by about 30 degrees from the horizontal plane by placing a bolster under it. The arms of the patient lie at the sides. This affords access to the left chest for the LIMA harvest. The left groin of the patient is always available for emergent institution of CPB if required. A perfusionist and a heart-lung machine are always on standby. A catheter is usually inserted into the left femoral artery in very obese patients to facilitate quick incorporation of CPB in an emergency situation. External pacing and defibrillator pads are also attached. Monitoring is performed with arterial and central venous catheters. The pulmonary artery catheter is placed only in patients with a left ventricular ejection fraction <30%. Changes in ST segment are monitored using a 5-channel electrocardiogram. The urinary bladder is catheterized. Transesophageal echocardiography is used in patients with poor left ventricular function or when a patient unexpectedly becomes hemodynamically unstable during the operative procedure. A warming blanket is always used and the room temperature is maintained at 22 ℃.
Operative steps
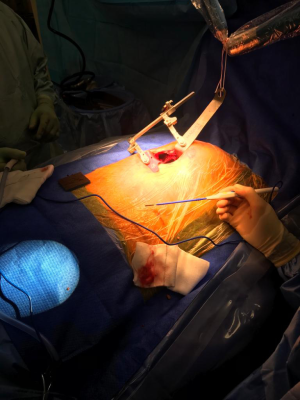
The incision for access is a 5–6 cm anterolateral muscle-sparing minithoracotomy, located 2–3 cm inferior to the nipple. The left hemithorax is entered through the 4th or 5th left intercostal space after the patient is connected to single lung ventilation. A specially designed IMA access retractor is used for IMA harvesting under direct vision (Figure 1). The technique of LIMA harvest, whether pedicled or skeletonized, is at the discretion of the operating surgeon. In obese patients the lateral pressure exerted on the wound with the retractor might cause necrosis and prompt infection in the edges of the wound. Similarly, females with large breast tissue are also at increased risk for wound necrosis and infection.In obese patients, the large amount of pericardial fat sometimes obscures the LIMA particularly in its proximal third. This can be overcome either by removal of this fat or by retraction of the fat inferiorly and laterally with a retraction suture. The whole length of the LIMA is harvested in most cases; however, in some patients with a long chest it may not be possible to take it down beyond the 2nd rib. Calafiore and colleagues have shown that 76.9% of the LADs needed a LIMA length of 9 cm or less to be grafted (11). Hence, the whole length of the LIMA is not necessary to reach the LAD in a MIDCAB procedure. Harvesting the distal LIMA segment is more important for its mobility to reach the LAD. The concern, that partial harvest of the LIMA may give rise to the possibility of competitive flow with the LAD, has also been put to rest by Luise and colleagues who showed that the flow reserve in the LIMA remained unchanged, even when it was partially harvested (14). The patient is then heparinised with 100–150 IU/kg of heparin. The activated clotting time is maintained at a level above 300 seconds throughout the operation. The pericardium is then opened longitudinally and the LAD is identified. The position of the LAD and its accompanying diagonal branches must be correlated with that on the angiography film. The distal end of the LIMA is transected only after the target vessel identified, is confirmed to be the LAD. Two 6.0 polypropelene sutures are used to hold the LIMA against the upper edge of the chest incision, following which, the distal end of the LIMA is prepared for anastomosis. The Octopus® Nuvo tissue stabilizer, a suction stabilizer for minimal access cardiac surgery, is used to stabilize the LAD. Two 6.0 polypropylene sutures are used retract the epicardial fat, if the LAD is deeply embedded in it. A 4.0 polypropylene pledgeted tourniquet is passed around the LAD proximal to the site of anastomosis or even a soft vascular bulldog can be used to occlude the flow. No ischemic preconditioning is used. Shunting of the LAD can be used alternatively (Table 3). Distal occlusion is preferably avoided. The anastomosis is performed using a single continuous 8-0 polypropylene suture, starting at the heel of the anastomosis. If the LIMA is pedicled, both sides of the pedicle are fixed to the epicardium once the anastomosis is completed. The flow is checked with a commercially available Doppler flow probe following release of the bulldog on the LIMA. Once haemostasis is confirmed, heparin is reversed with protamine. The pericardium is usually closed around the apex. The rest of the defect is closed by approximating the pericardial fat with the medial edge of the pericardium, so as to cover the distal segment of the LIMA. This protects the LIMA from being pushed anteriorly by the left lung upon inflation. A single chest drain is inserted into the left pleural cavity. The anaesthetist is then asked to gently inflate the left lung. At this stage, it is extremely important to prevent the lung from pushing the LIMA anteriorly, in order to prevent an avulsion. The LIMA should lie medial to the lung after it is completely inflated. An intercostal nerve block is then administered and the spread ribs are approximated. The thoracotomy is then closed in layers.
Table 3
| Presence of ischemic changes on ECG tracings on the monitor |
| Excessive retrograde flow of blood from the distal LAD |
| Large LAD with a lower grade stenosis (60–70%) |
| Proximal anastomosis in a large LAD |
MIDCAB, minimally invasive direct coronary artery bypass; LAD, left anterior descending artery.
Patients, who become extremely unstable during the course of the operation and develop low output syndrome refractory to medical management, are immediately connected to CPB by cannulation of the left femoral vessels. Indications for conversion to full sternotomy are listed in Table 4.
Table 4
| LIMA injury |
| LIMA too short |
| LAD is not visible along its entire length |
| LAD is heavily calcified |
| Anastomotic problems |
| Right ventricle injuries |
| Failure to tolerate single-lung ventilation |
MIDCAB, minimally invasive direct coronary artery bypass; LAD, left anterior descending artery; LIMA, left internal mammary artery.
Postoperative management
Postoperative management is similar that for other off-pump CABG patients. MIDCAB patients are usually good candidates for the fast-track recovery concept. The patient can be extubated in the operating room itself or shortly thereafter in the intensive care unit. Prompt extubation, early ambulation and aggressive physiotherapy are crucial to avoid postoperative atelectasis of the left lung, especially during the initial recovery phase. Good analgesia is important as some studies published in literature have shown that patients experience more pain with an anterolateral minithoracotomy as compared to sternotomy, especially during the first three days after surgery (15).
Outcomes
MIDCAB procedure is generally regarded as a demanding operation with a substantial learning curve (2). However, most published reports suggest excellent outcomes for this procedure. This phenomenon could be explained by the fact that majority of these publications emerge from high volume centres with experienced surgeons. The following subsections give a brief overview of the short- and long-term outcomes of isolated MIDCAB surgery as well as comparative outcomes with full-sternotomy isolated LAD revascularization and PCI of proximal LAD.
Outcomes of isolated MIDCAB
The first comprehensive evaluation of MIDCAB was published by Kettering and colleagues in 2004 (16). They undertook a systematic review of 15 studies published between 1998 and 2002 evaluating early and late mortality, intra- and postoperative complications, conversions, length of stay and analysis of graft occlusion or stenosis by either angiography or non-invasive tests after MIDCAB. These studies reporting earlier experience of MIDCAB revealed 0% to 4.9% early mortality and 0.3% to 12.6% late mortality (>30 days after MIDCAB). Non-fatal myocardial infarction rates ranged between 0% and 3.1%. The rates for intra- and postoperative complications (wound infections, reoperation for management of bleeding, arrhythmias, stroke, etc.) ranged between 1.6–40%. Conversion to sternotomy/CPB was reported to vary between 0% and 6.2%. Up to 8.9% patients required reintervention (repeat surgery or PCI) due to graft failure. This systematic review validated the safety and efficacy of MIDCAB and its results were further substantiated by an updated meta-analysis of 17 studies published by Kettering in 2008 (17).
Holzhey and associates published their experience of 1768 MIDCAB operations focusing on long-term outcome with more than 10 years of follow-up (18). The mean age of the study cohort was 63.4±10.8 years with mean ejection fraction of 60.0%±14.2%. Intraoperative conversion was necessary in 31 patients (1.75%). Fifteen patients (0.8%) died in the early postoperative period while 7 patients (0.4%) had a perioperative stroke. Early graft patency was reported 95.5% for the 712 patients that had postoperative angiography. A total of 59 patients (3.3%) needed short-term target vessel reintervention (48 re-operation, 11 PCI). Kaplan-Meier survival rate of 88.3% [95% confidence interval (CI), 86.6–89.9%] and 76.6% (95% CI, 73.5–78.7%) was reported at 5 and 10 years, respectively. The freedom from major adverse cardiac and cerebrovascular events (MACCE) and angina was 85.3% (95% CI, 83.5–87.1%) and 70.9% (95% CI, 68.1–73.7%) at 5 and 10 years, respectively. Similar outcomes were reported recently by Repossini and associates (19).
Comparison with full-sternotomy isolated LAD grafting
Stanbridge and Hadjinikolaou published a meta-analysis of early studies comparing 3,304 cases of MIDCAB and 3,060 cases of off-pump coronary artery bypass (OPCAB) surgery through a sternotomy (20). The early or late death rates between the two groups were similar (1.6% vs. 2.2%). The myocardial infarction rate (2.9% vs. 1.45%; P<0.03) and graft stenosis rate (6.6% vs. 1.4%; P<0.001) was significantly more in the MIDCAB cohort with similar length of stay (4.6 days), incidence of atrial fibrillation (9%), or conversion rate. Vicol et al. in their single centre series reported similar outcomes concluding that MIDCAB is technically more challenging than OPCAB and should therefore be performed by experienced surgeons on selected patients (21).
Birla and colleagues published a comparative analysis of 74 MIDCAB and 78 single vessel OPCAB graft procedures through a standard median sternotomy (22). They reported no statistically significant difference in the two groups in terms of mortality, recurrent myocardial infarction, postoperative stroke, wound infection, atrial fibrillation or need for reintervention. The conversion rate for MIDCAB was 8.1% with a significantly reduced hospital stay in the MIDCAB population (6.1 vs. 8.5 days, P<0.05).
Another large study with the longest follow-up comparing MIDCAB (n=508) and full sternotomy (n=160) CABG for isolated proximal LAD stenosis (23) reported similar 30-day mortality (2.0% vs. 2.5%), stroke rate (1.3% vs. 1.4%), and repeat revascularization rate (0.8% vs. 1.3%). At a mean follow-up time of 12.95±0.45 years, the long-term survival was comparable for the two groups. The late mortality for the entire cohort was 153 deaths with 40 (25%) in the full sternotomy group and 113 (22.24%) in MIDCAB group (P=0.64).
Comparison with percutaneous coronary intervention
Goy and colleagues were the first to report a comparison of MIDCAB and PCI in the randomized setting (24). They randomly assigned 68 patients to PCI and 66 patients to MIDCAB. The patients undergoing PCI had more recurrence of angina and at median follow-up of 2.5 years, freedom from adverse events was 86% for MIDCAB and 43% for PCI (P<0.01; relative risk 2.0 (95% CI, 1.7–2.3).
Contrary to this randomized controlled trial (RCT) of Goy and associates, Mariani and associates reported significantly less need for repeated revascularization (MIDCAB 96.9%±0.2% vs. angioplasty 67.6%±0.5%; P<0.001), and therefore the use of health care resources, with MIDCAB than with PCI in patients with isolated type C stenosis of the LAD (25).
Over the last 2 decades several observational studies, randomized controlled trials and meta-analysis have compared outcomes of MIDCAB and PCI using bare metal stents (26-32) (Table 5) as well as drug-eluting stents (34-45) (Table 6). The studies comparing MIDCAB and PCI with bare metal stents reported similar overall mortality and myocardial infarction rates but significantly lower rates of repeat revascularization and recurrence of angina with MIDCAB (26-33,46).
Table 5
| Variable | Cisowski et al. (26) 2002 | Drenth et al. (27) 2002a | Iakovou et al. (28) 2002 | Diegler et al. (29) 2002b | Reeves et al. (30) 2004 | Shirai et al. (31) 2004 | Kim et al. (32) 2005 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMS | MIDCAB | BMS | MIDCAB | BMS | MIDCAB | BMS | MIDCAB | BMS | MIDCAB | BMS | MIDCAB | BMS | MIDCAB | |||||||
| Country | Poland | Netherlands | United States | Germany | United Kingdom | United States | South Korea | |||||||||||||
| Study design | RCT | RCT | Prospective OBS | RCT | RCT | Prospective OBS | RCT | |||||||||||||
| Duration | 12 months | 48 months | 39 months | 6 months | 12 months | 92 months | 24 months | |||||||||||||
| No. of pts. | 50 | 50 | 51 | 51 | 441 | 119 | 110 | 110 | 50 | 50 | 429 | 152 | 50 | 50 | ||||||
| Age, years | 53±10 | 54±9.1 | 61±1.3 | 60±1.6 | 63±12 | 62±12 | 62±10.2 | 61±10 | 54 (49–61)c | 58 (53–67)c | 63±11 | 61±12 | 61±12 | 63±12 | ||||||
| Men | 84 | 82 | 75 | 78 | 68 | 71 | 72 | 77 | 86 | 70 | 65 | 73 | 60 | 70 | ||||||
| DM | 8 | 6 | 18 | 24 | 22 | 17 | 34 | 25 | – | – | 24 | 26 | 20 | 15 | ||||||
| HTN | 52 | 56 | 33 | 16 | 54 | 55 | 72 | 71 | – | – | 53 | 59 | 55 | 55 | ||||||
| Smokers | 52 | 48 | 30 | 37 | 46 | 56 | 25 | 25 | – | – | 19 | 15 | 45 | 55 | ||||||
| Prior AMI | – | – | 18 | 24 | 22 | 22 | 45 | 45 | – | – | 43 | 37 | 22 | 22 | ||||||
| Unstable angina | 10 | 8 | – | – | 68 | 60 | – | – | – | – | – | – | 65 | 55 | ||||||
| EF | – | – | – | – | 52±12 | 48±7 | 62±15 | 63±11 | – | – | 51±11 | 53±11 | 51±11 | 49±13 | ||||||
| MIDCAB technique | Thoracotomy | Thoracotomy | Exact method not specified | Thoracotomy | Thoracotomy | Exact method not specified | Ministernotomy | |||||||||||||
| Key outcome | MIDCAB superior to PCI | MIDCAB better than PCI | MIDCAB lower TVR and MACE than PCI | MIDCAB lower TVR and MACE than PCI | MIDCAB more cost; same efficacy as PCI | MIDCAB lower TVR than PCI | MIDCAB same efficacy as PCI | |||||||||||||
Values are percent, unless otherwise indicated. a, Drenth, 2004, presented updated 4-year results of the same randomized controlled trial. b, Thiele, 2005, presented updated 5-year results of the same randomized controlled trial. c, age is median (range). AMI, acute myocardial infarction; BMS, bare metal stent; DM, diabetes mellitus; EF, ejection fraction; HTN, hypertension; MACE, major adverse cardiac event; MIDCAB, minimally invasive coronary artery bypass graft surgery; OBS, observational; PCI, percutaneous coronary intervention; pts., patients; RCT, randomized controlled trial; TVR, target vessel revascularization. Reprinted from (33), with permission from Elsevier.
Table 6
| First author [year] | Country | Study design | Study period | Total participants (% male) | Mean age | Revascularization strategies | Follow-up | Key results (MIDCAB vs. DES) |
|---|---|---|---|---|---|---|---|---|
| Iqbal (34) [2017] | UK | Retrospective | 2004–2015 | 3,473 (79.1%) | 63 | MIDCAB vs. FDESa; MIDCAB vs. SDESb | 3 years | Similar mortality |
| Blazek (35) [2015] | Germany | RCT | 2003–2014 | 129 (70%) | 66 | MIDCAB vs. FDESc | 7.3 years | Lower composite outcome and TVR with MIDCAB |
| Hannan (36) [2014] | USA | Retrospective | 2008–2011 | 1,430 (66%) | NR | MIDCAB vs. DES* | 3 years | Similar composite outcome and lower TVR with MIDCAB |
| Benedetto (37) [2014] | UK | Retrospective | 2001–2013 | 606 (83%) | NR | MIDCAB vs. DES* | 2232 days | Lower mortality and TVR with MIDCAB |
| Ungureanu (38) [2013] | Belgium | Retrospective | NR | 204 (NR) | NR | MIDCAB vs. SDESd | 2 years | Lower TVR with MIDCAB |
| Jones (39) [2011] | UK | Retrospective | 2003–2010 | 874 (NR) | NR | MIDCAB vs. DES* | 4 years | Lower MACE, mortality and TVR with MIDCAB |
| Buszman (40) [2011] | Poland | Retrospective | 2004–2009 | 463 (75%) | 61 | MIDCAB vs. FDESa; MIDCAB vs. SDESb | 5 years | Similar MACCE, mortality, MI and lower TVR with MIDCAB |
| Patsa (41) [2010] | Greece | Retrospective | NR | 412 (NR) | NR | MIDCAB vs. FDESa; MIDCAB vs. SDESb | 26 months | Similar mortality, MI and lower TVR with MIDCAB |
| Thiele (42) [2009] | Germany | RCT | 2003–2007 | 130 (70%) | 66 | MIDCAB vs. FDESc | 12 months | Similar MACE, more MI and lower TVR with MIDCAB |
| Glineur (43) [2009] | Belgium | Retrospective | NR | 350 (NR) | 63 | MIDCAB vs. DES* | 2 years | Similar mortality and lower MACCE, MI, TVR with MIDCAB |
| Toutouzas (44) [2007] | Greece | Retrospective | 2001–2006 | 257 (86%) | 61 | MIDCAB vs. FDESa; MIDCAB vs. SDESe | 18 months | Similar MACE, mortality, MI and lower TVR with MIDCAB |
| Hong (45) [2005] | South Korea | RCT | 2003 | 189 (64%) | 61 | MIDCAB vs. FDESa | 6 months | Similar mortality, more MI and lower TVR with MIDCAB |
*, drug-eluting stent type not specified; a, sirolimus-eluting stents & paclitaxel-eluting stents; b, everolimus-eluting stents & zotarolimus-eluting stents; c, sirolimus-eluting stents; d, everolimus-eluting stents; e, ABT-578-eluting stents. DES, drug-eluting stent; FDES, first generation drug-eluting stent; MACE, major adverse cardiovascular events; MACCE, major adverse cardiovascular or cerebrovascular event; MI, myocardial infarction; MIDCAB, minimally invasive direct coronary artery bypass; NR, not reported; RCT, randomized controlled trial; SDES, second generation drug-eluting stent; TVR, target vessel revascularization.
The choice of optimal revascularization strategy for patients with isolated disease of the LAD remains a controversial issue in the current era of drug-eluting stents. The latest meta-analysis addressing this issue enrolled 7,710 participants from three RCTs and nine cohort studies (47). This meta-analysis validated the efficacy of both MIDCAB and drug-eluting stents as strategies for management of isolated LAD stenosis. However, target vessel revascularization rate was significantly higher with drug-eluting stents compared to MIDCAB. The findings of this meta-analysis endorse a significant increase in adverse events and target vessel revascularization previously reported by Kinnaird and associates in their meta-analysis (48).
Conclusions
MIDCAB surgery is a very attractive operation for revascularization of isolated LAD stenosis as it offers the benefits of the gold standard LIMA to LAD anastomosis accompanied by excellent cosmesis and a rapid recovery. Although, it is a challenging operation with a steep learning curve, it can be performed very elegantly and effectively by highly experienced surgeons. The reported short-, mid and long-term outcomes are excellent and validate the safety and efficacy of MIDCAB.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal for the series “Coronary Artery Bypass Grafting”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj.2020.03.05/coif). The series “Coronary Artery Bypass Grafting” was commissioned by the editorial office without any funding or sponsorship. SGR served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of AME Medical Journal from September 2019 to September 2021. The authors has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Varnauskas E. Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N Engl J Med 1988;319:332-7. [Crossref] [PubMed]
- Holzhey DM, Jacobs S, Walther T, et al. Cumulative sum failure analysis for eight surgeons performing minimally invasive direct coronary artery bypass. J Thorac Cardiovasc Surg 2007;134:663-9. [Crossref] [PubMed]
- Vineburg A. The treatment of angina pectoris by internal mammary artery implantation supplemented by pericardial fat wrap; covering four years clinical and eight years experimental experience. Conn State Med J 1955;19:281-302. [PubMed]
- Goetz RH, Rohman M, Haller JD, et al. Internal mammary-coronary artery anastomosis. A nonsuture method employing tantalum rings. J Thorac Cardiovasc Surg 1961;41:378-86. [PubMed]
- Olearchyk AS, Vasilii I. Kolesov. A pioneer of coronary revascularization by internal mammary-coronary artery grafting. J Thorac Cardiovasc Surg 1988;96:13-8. [Crossref] [PubMed]
- Kolesov VI. Kardiologiia 1967;7:20-5. [Initial experience in the treatment of stenocardia by the formation of coronary-systemic vascular anastomoses]. [PubMed]
- Garrett HE, Dennis EW, DeBakey ME. Aortocoronary bypass with saphenous vein graft. Seven-year follow-up. JAMA 1973;223:792-4. [Crossref] [PubMed]
- Trapp WG, Bisarya R. Placement of coronary artery bypass graft without pump oxygenator. Ann Thorac Surg 1975;19:1-9. [Crossref] [PubMed]
- Ankeney JL. Off-pump bypass surgery: the early experience, 1969-1985. Tex Heart Inst J 2004;31:210-3. [PubMed]
- Benetti FJ, Ballester C, Sani G, et al. Video assisted coronary bypass surgery. J Card Surg 1995;10:620-5. [Crossref] [PubMed]
- Calafiore AM, Giammarco GD, Teodori G, et al. Left anterior descending coronary artery grafting via left anterior small thoracotomy without cardiopulmonary bypass. Ann Thorac Surg 1996;61:1658-63. [Crossref] [PubMed]
- Subramanian VA. Less invasive arterial CABG on a beating heart. Ann Thorac Surg 1997;63:S68-71. [Crossref] [PubMed]
- Jacobs S, Holzhey D, Falk V, et al. High-risk patients with multivessel disease--is there a role for incomplete myocardial revascularization via minimally invasive direct coronary artery bypass grafting? Heart Surg Forum 2007;10:E459-62. [Crossref] [PubMed]
- Luise R, Teodori G, Di Giammarco G, et al. Persistence of mammary artery branches and blood supply to the left anterior descending artery. Ann Thorac Surg 1997;63:1759-64. [Crossref] [PubMed]
- Diegeler A, Walther T, Metz S, et al. Comparison of MIDCAP versus conventional CABG surgery regarding pain and quality of life. Heart Surg Forum 1999;2:290-5. [PubMed]
- Kettering K, Dapunt O, Baer FM. Minimally invasive direct coronary artery bypass grafting: a systematic review. J Cardiovasc Surg (Torino) 2004;45:255-64. [PubMed]
- Kettering K. Minimally invasive direct coronary artery bypass grafting: a meta-analysis. J Cardiovasc Surg (Torino) 2008;49:793-800. [PubMed]
- Holzhey DM, Cornely JP, Rastan AJ, et al. Review of a 13-year single-center experience with minimally invasive direct coronary artery bypass as the primary surgical treatment of coronary artery disease. Heart Surg Forum 2012;15:E61-8. [Crossref] [PubMed]
- Repossini A, Di Bacco L, Nicoli F, et al. Minimally invasive coronary artery bypass: Twenty-year experience. J Thorac Cardiovasc Surg 2019;158:127-38.e1. [Crossref] [PubMed]
- Stanbridge RD, Hadjinikolaou LK. Technical adjuncts in beating heart surgery comparison of MIDCAB to off-pump sternotomy: a meta-analysis. Eur J Cardiothorac Surg 1999;16:S24-33. [PubMed]
- Vicol C, Nollert G, Mair H, et al. Midterm results of beating heart surgery in 1-vessel disease: minimally invasive direct coronary artery bypass versus off-pump coronary artery bypass with full sternotomy. Heart Surg Forum 2003;6:341-4. [PubMed]
- Birla R, Patel P, Aresu G, Asimakopoulos G. Minimally invasive direct coronary artery bypass versus off-pump coronary surgery through sternotomy. Ann R Coll Surg Engl 2013;95:481-5. [Crossref] [PubMed]
- Raja SG, Garg S, Rochon M, et al. Short-term clinical outcomes and long-term survival of minimally invasive direct coronary artery bypass grafting. Ann Cardiothorac Surg 2018;7:621-7. [Crossref] [PubMed]
- Goy JJ, Eeckhout E, Burnand B, et al. Coronary angioplasty versus left internal mammary artery grafting for isolated proximal left anterior descending artery stenosis. Lancet 1994;343:1449-53. [Crossref] [PubMed]
- Mariani MA, Boonstra PW, Grandjean JG, et al. Minimally invasive coronary artery bypass grafting versus coronary angioplasty for isolated type C stenosis of the left anterior descending artery. J Thorac Cardiovasc Surg 1997;114:434-9. [Crossref] [PubMed]
- Cisowski M, Drzewiecki J, Drzewiecka-Gerber A, et al. Primary stenting versus MIDCAB: preliminary report-comparision of two methods of revascularization in single left anterior descending coronary artery stenosis. Ann Thorac Surg 2002;74:S1334-9. [Crossref] [PubMed]
- Drenth DJ, Winter JB, Veeger NJ, et al. Minimally invasive coronary artery bypass grafting versus percutaneous transluminal coronary angioplasty with stenting in isolated high-grade stenosis of the proximal left anterior descending coronary artery: six months' angiographic and clinical follow-up of a prospective randomized study. J Thorac Cardiovasc Surg 2002;124:130-5. [Crossref] [PubMed]
- Iakovou I, Dangas G, Mehran R, et al. Minimally invasive direct coronary artery bypass (MIDCAB) versus coronary artery stenting for elective revascularization of the left anterior descending artery. Am J Cardiol 2002;90:885-7. [Crossref] [PubMed]
- Diegeler A, Thiele H, Falk V, et al. Comparison of stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery. N Engl J Med 2002;347:561-6. [Crossref] [PubMed]
- Reeves BC, Angelini GD, Bryan AJ, et al. A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery. Health Technol Assess 2004;8:1-43. [Crossref] [PubMed]
- Shirai K, Lansky AJ, Mehran R, et al. Minimally invasive coronary artery bypass grafting versus stenting for patients with proximal left anterior descending coronary artery disease. Am J Cardiol 2004;93:959-62. [Crossref] [PubMed]
- Kim JW, Lim DS, Sun K, et al. Stenting or MIDCAB using ministernotomy for revascularization of proximal left anterior descending artery? Int J Cardiol 2005;99:437-41. [Crossref] [PubMed]
- Deo SV, Sharma V, Shah IK, et al. Minimally invasive direct coronary artery bypass graft surgery or percutaneous coronary intervention for proximal left anterior descending artery stenosis: a meta-analysis. Ann Thorac Surg 2014;97:2056-65. [Crossref] [PubMed]
- Iqbal MB, Ilsley C, De Robertis F, et al. Comparison of outcomes of coronary artery bypass grafting using internal mammary graft versus percutaneous coronary intervention for isolated proximal left anterior descending narrowing. Am J Cardiol 2017;119:719-26. [Crossref] [PubMed]
- Blazek S, Rossbach C, Borger MA, et al. Comparison of sirolimus-eluting stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery: 7-year follow-up of a randomized trial. JACC Cardiovasc Interv 2015;8:30-8. [Crossref] [PubMed]
- Hannan EL, Zhong Y, Walford G, et al. Coronary artery bypass graft surgery versus drug-eluting stents for patients with isolated proximal left anterior descending disease. J Am Coll Cardiol 2014;64:2717-26. [Crossref] [PubMed]
- Benedetto U, Raja SG, Soliman RFHarefield Cardiac Outcomes Research Group, et al. Minimally invasive direct coronary artery bypass improves late survival compared with drug-eluting stents in isolated proximal left anterior descending artery disease: a 10-year follow-up, single-center, propensity score analysis. J Thorac Cardiovasc Surg 2014;148:1316-22. [Crossref] [PubMed]
- Ungureanu C, Laruelle C, Khoury E, et al. Retrospective comparison of minimally invasive direct coronary artery bypass surgery versus Xience drug eluting stents in isolated proximal left anterior descending coronary artery stenosis. Acta Cardiol 2013;68:104-5.
- Jones D, Rathod K, Gallagher S, et al. Drug eluting stents in the treatment of isolated proximal LAD disease are associated with similar outcomes compared to minimally invasive LIMA grafts. J Am Coll Cardiol 2011;58:B56. [Crossref]
- Buszman P, Król L, Cisowski M, et al. DES vs. MIDCAB for proximal LAD disease: long term registry results. J Am Coll Cardiol 2011;58:20:B53.
- Patsa C, Toutouzas K, Tsiamis E, et al. Long term clinical outcome after DES implantation or LIMA grafting in patients with an isolated pLAD lesion. Eur Heart J 2010;31:19.
- Thiele H, Neumann-Schniedewind P, Jacobs S, et al. Randomized comparison of minimally invasive direct coronary artery bypass surgery versus sirolimus-eluting stenting in isolated proximal left anterior descending coronary artery stenosis. J Am Coll Cardiol 2009;53:2324-2331. [Crossref] [PubMed]
- Glineur D, Boodhwani M, Noirhomme P, et al. Short and midterm clinical outcome following single vessel LAD revascularization with MIDCAB versus DES. ISMICS 2009;3:159.
- Toutouzas K, Patsa C, Vaina S, et al. Drug eluting stents versus coronary artery bypass surgery in patients with isolated proximal lesion in left anterior descending artery suffering from chronic stable angina. Catheter Cardiovasc Interv 2007;70:832-7. [Crossref] [PubMed]
- Hong SJ, Lim DS, Seo HS, et al. Percutaneous coronary intervention with drug-eluting stent implantation vs. minimally invasive direct coronary artery bypass (MIDCAB) in patients with left anterior descending coronary artery stenosis. Catheter Cardiovasc Interv 2005;64:75-81. [Crossref] [PubMed]
- Jaffery Z, Kowalski M, Weaver WD, et al. A meta-analysis of randomized control trials comparing minimally invasive direct coronary bypass grafting versus percutaneous coronary intervention for stenosis of the proximal left anterior descending artery. Eur J Cardiothorac Surg 2007;31:691-7. [Crossref] [PubMed]
- Raja SG, Uzzaman M, Garg S, et al. Comparison of minimally invasive direct coronary artery bypass and drug-eluting stents for management of isolated left anterior descending artery disease: a systematic review and meta-analysis of 7,710 patients. Ann Cardiothorac Surg 2018;7:567-76. [Crossref] [PubMed]
- Kinnaird T, Kwok CS, Narain A, et al. Meta-Analysis of Percutaneous coronary intervention with drug-eluting stent versus coronary artery bypass grafting for isolated proximal left anterior descending coronary disease. Am J Cardiol 2016;118:1171-7. [Crossref] [PubMed]
Cite this article as: Garg S, Raja SG. Minimally invasive direct coronary artery bypass (MIDCAB) grafting. AME Med J 2020;5:19.