
Surgical management of the localized renal mass: risk and benefit trade-offs and surgical approach considerations
Introduction
Greater than 70,000 patients are diagnosed with kidney cancer annually in the United States, with an associated approximately 15,000 kidney cancer-related deaths (1). There has been a significant increase in disease incidence over recent decades, driven largely by increased utilization of cross-sectional imaging and longer life expectancies (2,3). Greater than 50% of renal masses are now diagnosed incidentally, which has led to a “stage migration” where earlier diagnosis of smaller masses has led to a shift towards lower staging (4,5). Currently, the majority of patients are diagnosed with clinically localized disease (stages I–II), and thus have favorable survival estimates at 80–90% (6).
Renal masses include a diverse group of tumors comprised of benign masses, indolent cancers, and more aggressive cancers. Over 90% of kidney cancers are renal cell carcinoma (RCC). While advances have been made in systemic therapies, the mainstay of treatment for the localized renal mass remains surgical resection, either via radical nephrectomy (RN) or partial nephrectomy (PN). While RN has served as the definitive management option over the past century, the utilization of PN has been rapidly increasing over the last two decades, especially in tertiary academic centers (7). Factors contributing to this shift include increased prevalence of smaller tumors, advances in surgical technology, and improved understanding of renal surgery’s impact on functional kidney outcomes (8).
Despite the increased utilization of PN, there continues to be a lack of high-quality, prospective evidence to help inform the selection of PN vs. RN as treatment for clinically localized disease. Treatment decision-making thus relies primarily on observational-based data, clinical practice guidelines, and shared decision-making. Guidelines currently recommend PN over RN for T1a tumors amenable to nephron-sparing surgery (NSS) (6,9). For T1b and T2 tumors, PN is supported as an option in select patients (6,9). Understanding the risk and benefit tradeoffs for PN vs. RN for T1b and T2 tumors is critical in optimally treating patients with various age- and lifestyle-related comorbidities. Recent advances in surgical technology and technique also influence a urologist’s approach to treating localized kidney cancer. Herein, we set out to first review the latest evidence on the risk and benefit tradeoffs for PN vs. RN, and to provide an overview of recent considerations in surgical technique in order to best inform treatment decision-making for the localized renal mass.
Treatment decision-making between PN versus RN
Effect of PN on postoperative renal function
The most clearly established benefit of PN as compared to RN is improved long-term postoperative renal function. PN was initially developed as an option for patients with intrinsic renal deficits such as solitary kidney, bilateral renal tumors, or pre-existing chronic kidney disease (CKD). Over time, surgeons began implementing PN in patients without pre-existing renal deficit. Retrospective studies were the first to show that PN can be safely used to preserve renal function in patients with normal contralateral kidneys. A 2011 meta-analysis of 31,729 patients undergoing RN and 9,281 undergoing PN found that those who underwent PN were significantly less likely to develop CKD (hazard ratio 0.39, 95% confidence interval, 0.33–0.47) (10). A more recent 2017 meta-analysis examining larger tumors (clinical stage T1b and T2) found comparable results, with patients undergoing PN having a lower risk of developing CKD (11). PN has also been associated with a reduced risk of downstream adverse renal outcomes, with a 2008 retrospective cohort study finding that patients with T1a tumors who underwent PN were less likely to require dialysis or kidney transplant (12). PN has also been associated with potential benefits in other organ systems outside of the genitourinary system, with retrospective studies reporting decreased rates of anemia of chronic disease, less utilization of erythropoiesis-stimulating agents, and even reduced rates of osteoporosis and fractures in patients undergoing PN as compared to RN (13-15).
While the vast majority of literature comparing PN vs. RN has been retrospective or observational in nature, there has been one prospective, randomized controlled trial (RCT) to date in this space, the European Organization for Research and Treatment of Cancer (EORTC) trial 30904. The central findings of this study will be discussed further in the next section, however, it is important to note here that on secondary analysis it did confirm PN to be associated with improved renal functional outcomes (12). In this multi-centered RCT, patients with a renal mass <5 cm and an anatomically normal contralateral kidney were randomized to RN (n=268) and PN (n=273) with the primary endpoint of all-cause mortality (ACM). PN was associated with a lower risk of developing CKD than RN (64.7% vs. 85.7%) at a median follow up of 6.7 years. Despite a lower risk of CKD, notably they did not find a statistically lower risk of full renal failure, defined as estimated glomerular filtration rate (eGFR) <15 mL/min/1.732 (16).
It has been suggested that this lack of significance of risk of renal failure in EORTC 30904 may be in part attributable to the inclusion criteria of an anatomically normal contralateral kidney. Studies have shown that a healthy solitary kidney is generally sufficient to maintain normal renal function over the long term, a concept for which strong support can be found in the transplant literature (17). For example, one large cohort study of kidney donors showed that, although the risk of renal failure 15 years after donation was approximately eight times higher than that of matched, healthy counterparts, the absolute risk for these donors remained very low, approximately 0.3% (18). That a solitary healthy kidney in this population can maintain normal renal function over the long term would suggest that it potentially may be difficult to detect clinically significant changes in renal function after PN vs. RN. Of course, the generally young and healthy kidney donor population differs from the localized renal mass population, that tends to be older, more comorbid, and more likely to have preexisting CKD (8,19). Nonetheless, that an otherwise healthy preexisting kidney is very often sufficient to maintain normal renal function presents a key challenge in detecting differences in clinically significant harm in RN vs. PN (17).
Effect of PN on survival outcomes
Despite PN’s clear benefits regarding post-operative renal function, PN’s effect on survival outcomes has been less clear. Many initially presumed that the reduced risk of developing CKD would translate to improved overall survival (OS) in patients with normal baseline kidney function. Indeed, the first single-institution retrospective studies, followed by more recent and extensive meta-analyses, have reported PN to be associated with a survival benefit (11,20,21). For instance, a 2012 systematic review and meta-analysis of 21 studies found PN to be correlated with a 19% risk reduction in ACM (hazard ratio 0.81, P<0.0001) (21).
However, when assessing OS benefit, it is critical to note the concern for selection bias in retrospective studies, as individual tumor characteristics inevitably inform the surgeon’s decision between PN and RN. Evidence for this bias is supported by the paradoxical finding in several systematic reviews and meta-analyses that PN is unexpectedly associated with superior cancer-specific mortality as compared to RN, a finding that biologically and intuitively does not make sense given the inherently increased risk of positive margins in PN as compared to RN for the small renal mass (11,21). The rationale for this conclusion centers predominately around selection bias. First, surgeons are inherently more likely to treat favorably-located tumors with PN. Given that aggressive tumor biology correlates with more complex tumor anatomy, it stands that any retrospective PN cohort may have a different, perhaps less aggressive, tumor biology than that of its respective RN cohort (22). Another factor potentially contributing to this selection bias is that healthier, stronger patients are more likely to pursue the more complex, higher risk PN operation. As such, there are many inherent (and difficult to measure) confounders likely present in any retrospective comparison study of PN vs. RN, which theoretically would magnify the calculated survival effect of PN.
To adjust for this potential selection bias and minimize the confounding present in these observational studies, intricate statistical methodologies such as instrumental variable analysis have been utilized to “pseudo-randomize” patients. One such study examined 7,138 Medicare beneficiaries with clinical T1a renal tumors from 1992 to 2007 (23). After utilizing instrumental variable analysis in attempts to adjust for the confounders mentioned previously, they reported that PN was still associated with improved ACM (hazard ratio 0.54; 95% CI, 0.34–0.85) (23).
However, the validity of these purported survival benefits were immediately questioned when the results of EORTC 30904, the lone prospective level 1 evidence in this space as discussed earlier, were published. EORTC 30904 found, unexpectedly, that patients randomized to RN had improved survival as compared to those randomized to PN in their intention to treat analysis. This study’s strength is its prospective, randomized nature. However, despite this, there are also several limitations. The most notable was the challenging patient accrual that led to a significantly underpowered study, only enrolling 541 patients across 45 medical centers over ten years, far from their target of 1,300 patients. This equates to approximately one patient per year per institution. Given that many of these institutions are high-volume centers of excellence, only enrolling about one patient per year does limit the study’s generalizability. Also, there was significant cross-over following the randomization, potentially further limiting its conclusions (23).
Thus, regarding the potential survival benefits of PN, urologists are left with the challenge of reconciling the results of the lone RCT with those of the multitude of other observational studies: a task which has certainly muddied the waters. Unless other prospective trials emerge, it is unlikely that this literature will be decisively clarified anytime soon. One such potential trial has been proposed by Campbell and colleagues that would randomize patients with T1b or T2 tumors to either PN or RN and assess OS as the primary outcome, with cancer-specific mortality, renal function, cardiac, and metabolic outcomes as secondary outcomes (17,24).
Perioperative risks of PN versus RN
Although PN confers the potential benefits as described above, it is also an inherently more complex operation than RN. There are several reasons for this, including a more extensive dissection, incision into the highly vascular kidney parenchyma with subsequent renorrhaphy, and prolonged operative time. As such, decision-making must include a careful assessment of the marginal perioperative risks associated with PN as compared to RN in the context of each individual patient.
For one, PN is associated with a greater intraoperative blood loss and perioperative transfusion rate than RN (25,26). This finding has been widely supported in the literature, including a 2013 retrospective study using the Nationwide Inpatient Sample that found increased post-operative bleeding associated with PN as compared to RN (7.0% vs. 5.3%; P<0.001) (26). Data from the prospective RTC, EORTC 30904, also supports PN to be associated with higher rates of postoperative bleeding (13).
Additional perioperative risks of PN have been linked to tumor complexity, often characterized by nephrometry score. These risks include urine leak, renal artery aneurysm, and ureteral stricture, as well as increased operative time, hospital stay, and perioperative mortality (22-24). Risk of urine leak has been particularly well studied. A 2011 retrospective study found that each additional nephrometry score point corresponded with a 35% increased odds of developing a urine leak following PN (27). Another study specifically looking at outcomes of PN of larger T2 masses reported the risk of urine leak to be 17% (28). It does stand to reason that performing PN on a complex, endophytic tumor abutting the collecting system would necessarily increase the risk of urine leak. That said, it is important to note that the majority of urine leaks following PN are temporary and will resolve spontaneously with conservative management (29,30). Encouragingly, more recent reports suggest that the rates of urine leaks following PN may be decreasing with the adoption of the robotic platform (29).
Although the literature clearly supports increased perioperative risk associated with PN as compared to RN, it should be noted that overall, both operations are generally well-tolerated in correctly selected patients (31-33). In fact, one retrospective study of 1,092 patients found that a patient’s co-morbidity status, as assessed by Charlson comorbidity index, was a significant predictor of complications (31). Regardless of whether the patient underwent RN or PN, high-risk patients were twice as likely (16.1 vs. 8.1%, P<0.001) to have Clavien-III complications as compared to low-risk patients (31). These findings suggest that patients with fewer comorbidities, who may be better able to tolerate any increased risk of perioperative complications, are likely excellent candidates for PN.
Oncologic control of PN versus RN
The majority of the literature to date suggests that PN confers similar oncologic efficacy as compared to RN (11,17,34). However, similar to above discussions regarding OS, it is important to note that the current body of evidence is primarily retrospective, a limitation that leaves it subject to various biases. An example of this are recent meta-analyses that have found a paradoxically increased rate of cancer-specific survival in PN as compared to RN, a finding likely best explained by selection bias. This is to say that patients undergoing PN on average had different, or biologically less aggressive, tumors than those undergoing RN (11,21). Despite these limitations of the retrospective studies, it is encouraging that the only level I evidence to date, EORTC 30904, did report similar oncologic efficacy between PN and RN, finding no change in ten-year cancer progression between PN and RN (4.1% vs. 3.3%, respectively; P=0.48) (25). Future prospective trials would help to clarify this topic further.
Another critical oncologic issue is the inherent risk of positive surgical margins (PSM) after PN due to preservation of the surrounding parenchyma. The published rate of PSM after PN ranges between 0% and 7% (35). However, the significance of PSM in terms of oncologic impact is controversial. While initial studies were divided on the association of PSMs with recurrence and subsequent effect on survival, more recent studies have indeed found an association with decreased OS (36-39). Current efforts are focused on identifying risk factors for PSM after PN. A prospective, multicenter, observational study published in 2020 found the following independent predictors of PSM in PN: low-volume center, imperative (as compared to elective) indication, laparoscopic (as compared to open), presence of lymphovascular invasion, upstaging to pT3a, and clinical-stage cT1a vs. cT2 (38).
Impact of multifocality on PN versus RN
Another factor to consider in the decision-making process for PN vs. RN is the possibility of tumor multifocality. While there is much written about familial RCC and the increased risk of tumor multifocality, it should be noted that multifocal renal masses can also occur sporadically. Regardless of cause, reports on the incidence of renal tumor multifocality on presentation range from 5% to 11% (40-42). Several studies in the past decade have demonstrated the feasibility of PN for multifocal tumors (43,44). For example, a 2010 series of 58 patients undergoing PN for multifocal cT1b or greater tumors found similar rates of cancer-specific survival and OS to reported rates in the solitary renal mass population (43). As such, an additional ipsilateral or contralateral renal tumor at presentation, or the prospect of developing another tumor in the future, can undoubtedly make PN a more attractive option with goal of preserving as much renal function as possible.
Surgical approach considerations in RN
RN remains the gold standard treatment for the localized renal mass in any patient not suitable for NSS. Clayman et al. ushered in the minimally invasive era to renal surgery with the first laparoscopic RN (LRN) in 1991 (45). A laparoscopic approach allowed for equivalent oncologic outcomes as compared to open RN (ORN), but with faster recovery, lower blood loss, and decreased complication rates. Laparoscopy quickly became the preferred approach for patients with localized renal cancer requiring RN (46,47).
The emergence of the robotic platform in the early 2000s has again shifted the paradigm of surgical approach across urology. Primarily driven by the widespread adoption of robotic-assisted radical prostatectomy, the robotic surgical system is now at the forefront of minimally-invasive urologic surgery. This trend has also impacted RN as seen by the increasing utilization of robotic RN (RRN). Data from the Premier Healthcare database shows that for patients in the United States undergoing RN, the use of the robotic approach increased from 1.5% in 2003 to 27% in 2015 (48). Since 2009, decreases in LRN have paralleled the increases in RRN, and LRN was overtaken by RRN for the first time in 2015.
In contrast to other urologic operations, however, for RN the robotic approach shows no clear benefit as compared to a pure laparoscopic approach (49,50). One plausible explanation is that standard RN does not entail any intracorporeal suturing, a primary advantage of the robotic approach in radical prostatectomy and PN (48). Further, studies have shown that as compared to LRN, RRN has increased costs and longer operative times, but equivalent oncologic outcomes (47,50,51).
With no clear benefits of RRN as compared to LRN, some have focused on the robotic platform’s increased costs, arguing that RRN represents a “technical overtreatment,” although exact estimates of cost differences vary (50). A 2014 meta-analysis reported that using LRN instead of RRN was associated with a $1,300 cost savings (50). However, a 2017 retrospective study used SEER data and reported an almost $10,000 difference in total inpatient charges after RRN vs. after LRN ($53,681 vs. $44,161, P<0.01) (49). Regardless of the exact amount, all agree that embracing the robotic platform for RN currently incurs a greater expense. Most of these cost increases have been attributed to longer OR times; however, some have also suggested that hospitals are simply more likely to raise charges to account for the higher costs, acquisition, and maintenance of the robotic system (48).
In the context of rising US health care costs, it is reasonable to promote choosing the less costly option where surgical approaches are otherwise equivalent (52). However, it is clear that the robotic platform offers clear advantages in other urologic operations and is here to stay, perhaps even becoming the centerpiece of contemporary urologic surgical education. New urologists completing their training may prefer robotic surgery over pure laparoscopy due to its shorter learning curve, ergonomic console, and perceived increased precision (48). From a cost point of view, one may argue that we are still early in the robotic era and that with further adoption of this technology will come decreased costs. This hypothesis holds especially true if operative times continue to decline as hospital team members become more facile with robotic platform logistics. All taken together, it stands to reason that in the face of equivalent outcomes data, as is the case for RRN and LRN, the urologic surgeon should choose the approach with which he or she is most comfortable and experienced with to safely and efficiently complete the operation.
Surgical approach considerations in PN
Emergence of robotic-assisted laparoscopic nephrectomy
The rise of the robotic platform has significantly changed the PN landscape, undoubtedly serving as one of the main factors driving the aforementioned trend of increasing PN utilization. Gettman et al. is credited with the first reported robotic-assisted PN (RPN) in 2004 (53). With magnified 3-dimensional visualization and articulating instruments to facilitate intracorporeal suturing, many view the robotic approach as significantly easier to learn and perform as compared to the pure laparoscopic approach (54). This “shorter learning curve” has helped bridge a technical gap in NSS, making minimally-invasive NSS more accessible to patients as more surgeons become comfortable with the robotic approach. Considering this, it is no surprise that RPN has continued to grow in popularity (55-57). One recent study found that among cT1 tumors undergoing PN, use of RPN increased from 41% in 2010 to 63% in 2013 (57).
Outcomes data for RPN compared to laparoscopic PN (LPN) and open PN (OPN) have been promising, showing RPN to be equivalent or superior to the other modalities. Although there is currently no prospective level I evidence in this space, there is an abundance of observational and retrospective studies reporting excellent outcomes data for RPN. A recent 2018 meta-analysis by Cacciamani et al. performed a pooled analysis of 33 studies totaling 20,282 patients, finding RPN superior to OPN for blood loss, transfusions, complications, hospital stay, readmissions, percent reduction of latest eGFR, overall mortality, and recurrence rate (58). The same study found that RPN was superior to LPN for ischemia time, conversion to open rate, intraoperative and postoperative complication rate, PSM, percentage decrease in eGFR, and overall mortality (58). The consensus of the best evidence to date suggests RPN to be at minimum equivalent, and likely superior, to other PN modalities.
Tumor enucleation (TE) versus PN
An interesting shift in PN resection technique has been the increasing utilization of TE and other related hybrid methods. Initially utilized in the familial RCC population as a method to preserve parenchyma in patients with multiple tumors, TE involves blunt separation of the natural plane between the tumor pseudocapsule and kidney parenchyma, and is depicted in Figure 1 (6,59-61). This method contrasts with the more traditional wedge resection technique which leaves a clear margin of renal parenchyma around the tumor specimen. As the body of literature investigating TE and associated hybrid approaches continues to grow, some have advocated for the development of a standardized reporting method for resection technique. Minervini and colleagues have proposed the Surface-Intermediate-Base (SIB) score framework, which entails a macroscopic quantification of the margins of normal parenchyma visible at the level of the superficial, intermediate, and base surfaces of the tumor (62,63). Adding up these values determines one of five potential technique variations; which range from pure enucleation (score sum 0–1), pure enucleoresection (score sum 3), hybrid enucleoresection (score sum 4), and resection (score sum 5) (62,64). While widespread adoption of a standardized reporting framework for resection technique during PN remains to be seen, the impact of these differing resection techniques remains controversial.
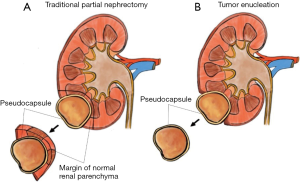
TE proponents argue there are several benefits to this resection method. By not entering the parenchyma, the surgeon can minimize hemorrhage while maximizing the preservation of remaining nephrons. Taking advantage of this natural, often avascular, plane facilitates blunt dissection and can make the surgery less technically challenging, lowering operative time and morbidity to the patient (59). Several retrospective studies have shown encouraging functional and oncologic outcomes associated with TE as compared to traditional PN with resection of visible parenchymal margin (59,60,65,66). A recent meta-analysis examined ten retrospective studies and three RCTs. The analysis found that when compared to conventional PN, TE was associated with shorter operative time (155 vs. 168 min), lower blood loss (182 vs. 259 mL), and smaller post-operative changes in eGFR (2.2 vs. 5.2 mL/min/1.73 mm2), with equivalent oncologic outcomes (no difference in PSM rate, recurrence rate, nor OS rate) (66).
Despite these encouraging findings, others urge caution in quickly embracing TE techniques for several reasons (41). Reports have shown that the pseudocapsule, which TE techniques are based on, is incomplete in up to 30% of cases (41,67). Further, another study found that the tumor may invade the pseudocapsule in up to 20% of cases, theoretically increasing the risk of a PSM (59). Despite several studies showing an equivalent PSM rate between TE and traditional PN, much of these rely on weak, retrospective data subject to selection bias. It stands to reason that tumors upon which a surgeon chooses to perform TE are likely smaller and more favorably-located as compared to those selected for traditional PN. For these reasons, clinical practice guidelines currently recommend TE be considered in patients with imperative indications—such as familial RCC, multifocal disease, or severe CKD—in efforts to optimize nephron preservation. The guidelines leave the rest to surgeon discretion, taking into consideration patient and tumor characteristics (6).
Role of renal mass biopsy (RMB)
When discussing the treatment considerations between PN vs. RN for the small renal mass it is also prudent to comment on RMB. While a full discussion of the strengths and limitations of RMB are outside the scope of this chapter, it is important to note there has been a recent push for urologists to increase utilization of RMB in patients in which it may affect management. This resurgence of RMB has been driven by improvements in technique, a relatively low procedural risk, and improved diagnostic rates (68,69). A recent meta analysis found RMB of small renal masses to have sensitivity and specificity of 99.7% and 98.2%, respectively, for diagnosis of malignancy (70). Further, RMB was shown to have strong concordance with tumor specimen for histologic tumor subtype, 90.3% (IQR: 84–94.4%) (70). However, the main limitation of RMB is its poor concordance with tumor grade, which this same meta analysis found to be only 66.7% (IQR 60–69.8%), a phenomenon that has been attributed to the marked tumor heterogeneity of renal malignancies (71).
In light of these considerations, it follows that for the appropriate patient RMB can be clinically valuable in helping inform the optimal treatment strategy. For example, much has been published on the use of RMB to assist in the decision between active surveillance vs. operative intervention (6). However, specifically for the purposes of this chapter, RMB also has potential to inform the optimal surgical approach after the decision for surgery has been made. For instance, one can imagine a middle age, comorbid patient with a complex, endophytic mass, in whom the RMB returns showing a more aggressive tumor, such as type 2 papillary RCC, may tip the scales away from PN (especially from TE approaches) and towards RN. In this way, RMB can clearly play a role informing surgical approach considerations for the localized renal mass in select patients.
Conclusions
The surgical approach to a localized renal mass requires a nuanced assessment of several factors, including the size, complexity, and oncologic potential of the tumor, combined with the overall health, renal function, and degree of comorbidities of the patient. Discussing these factors in the context of the relative efficacy and risks of PN and RN can inform a thoughtful shared decision-making process to determine the best approach for the patient.
Acknowledgments
Funding: Dr. Simon P. Kim is supported by an NIH R01 grant (MD12-003) and the Schramm Foundation.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal for the series “Surgical Management of Genitourinary Malignancies”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-77/coif). The series “Surgical Management of Genitourinary Malignancies” was commissioned by the editorial office without any funding or sponsorship. Dr. SPK served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of AME Medical Journal from September 2019 to August 2021. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kidney and Renal Pelvis Cancer - Cancer Stat Facts [cited 2019 Dec 30]. Available online: https://seer.cancer.gov/statfacts/html/kidrp.html
- Gandaglia G, Ravi P, Abdollah F, et al. Contemporary incidence and mortality rates of kidney cancer in the united states. Can Urol Assoc J 2014;8:247-52. [Crossref] [PubMed]
- Patel HD, Gupta M, Joice GA, et al. Clinical Stage Migration and Survival for Renal Cell Carcinoma in the United States. Eur Urol Oncol 2019;2:343-8. [Crossref] [PubMed]
- Kane CJ, Mallin K, Ritchey J, et al. Renal cell cancer stage migration. Cancer 2008;113:78-83. [Crossref] [PubMed]
- Rossi SH, Klatte T, Usher-Smith J, et al. Epidemiology and screening for renal cancer. World J Urol 2018;36:1341-53. [Crossref] [PubMed]
- Campbell S, Uzzo RG, Allaf ME, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. J Urol 2017;198:520-9. [Crossref] [PubMed]
- Thompson RH, Kaag M, Vickers A, et al. Contemporary use of partial nephrectomy at a tertiary care center in the United States. J Urol 2009;181:993-7. [Crossref] [PubMed]
- Huang WC, Donin NM, Levey AS, et al. Chronic Kidney Disease and Kidney Cancer Surgery: New Perspectives. J Urol 2020;203:475-85. [Crossref] [PubMed]
- Jonasch E, Agarwal N, Alva A, et al. Continue NCCN Guidelines Panel Disclosures NCCN Guidelines Version 2. 2020 Kidney Cancer 2019;
- Kim SP, Shah ND, Weight CJ, et al. Contemporary trends in nephrectomy for renal cell carcinoma in the united states: Results from a population based cohort. J Urol 2011;186:1779-85. [Crossref] [PubMed]
- Mir MC, Derweesh I, Porpiglia F, et al. Partial Nephrectomy Versus Radical Nephrectomy for Clinical T1b and T2 Renal Tumors: A Systematic Review and Meta-analysis of Comparative Studies. Eur Urol 2017;71:606-17. [Crossref] [PubMed]
- Miller DC, Schonlau M, Litwin MS, et al. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer 2008;112:511-20. [Crossref] [PubMed]
- Sun M, Bianchi M, Hansen J, et al. Chronic kidney disease after nephrectomy in patients with small renal masses: A retrospective observational analysis. Eur Urol 2012;62:696-703. [Crossref] [PubMed]
- Woldrich J, Mehrazin R, Bazzi WM, et al. Comparison of rates and risk factors for development of anaemia and erythropoiesis-stimulating agent utilization after radical or partial nephrectomy. BJU Int 2012;109:1019-25. [Crossref] [PubMed]
- Bagrodia A, Mehrazin R, Bazzi WM, et al. Comparison of rates and risk factors for development of osteoporosis and fractures after radical or partial nephrectomy. Urology 2011;78:614-9. [Crossref] [PubMed]
- Scosyrev E, Messing EM, Sylvester R, et al. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol 2014;65:372-7. [Crossref] [PubMed]
- Kim SP, Campbell SC, Gill I, et al. Collaborative Review of Risk Benefit Trade-offs Between Partial and Radical Nephrectomy in the Management of Anatomically Complex Renal Masses. Eur Urol 2017;72:64-75. [Crossref] [PubMed]
- Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA 2014;311:579-86. [Crossref] [PubMed]
- Capitanio U, Bensalah K, Bex A, et al. Epidemiology of Renal Cell Carcinoma. Eur Urol 2019;75:74-84. [Crossref] [PubMed]
- Lau WK, Blute ML, Weaver AL, et al. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc 2000;75:1236-42. [Crossref] [PubMed]
- Kim SP, Thompson RH, Boorjian SA, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol 2012;188:51-7. [Crossref] [PubMed]
- Smaldone MC, Churukanti G, Simhan J, et al. Clinical characteristics associated with treatment type for localized renal tumors: implications for practice pattern assessment. Urology 2013;81:269-75. [Crossref] [PubMed]
- Tan HJ, Norton EC, Ye Z, et al. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA 2012;307:1629-35. [Crossref] [PubMed]
- Weight CJ, Miller DC, Campbell SC, et al. The management of a clinical t1b renal tumor in the presence of a normal contralateral kidney. J Urol 2013;189:1198-202. [Crossref] [PubMed]
- Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011;59:543-52. [Crossref] [PubMed]
- Kim SP, Leibovich BC, Shah ND, et al. The relationship of postoperative complications with in-hospital outcomes and costs after renal surgery for kidney cancer. BJU Int 2013;111:580-8. [Crossref] [PubMed]
- Bruner B, Breau RH, Lohse CM, et al. Renal nephrometry score is associated with urine leak after partial nephrectomy. BJU Int 2011;108:67-72. [Crossref] [PubMed]
- Breau RH, Crispen PL, Jimenez RE, et al. Outcome of Stage T2 or Greater Renal Cell Cancer Treated With Partial Nephrectomy. J Urol 2010;183:903-8. [Crossref] [PubMed]
- Potretzke AM, Knight BA, Zargar H, et al. Urinary fistula after robot-assisted partial nephrectomy: a multicentre analysis of 1 791 patients. BJU Int 2016;117:131-7. [Crossref] [PubMed]
- Pierorazio PM, Johnson MH, Patel HD, et al. Management of Renal Masses and Localized Renal Cancer: Systematic Review and Meta-Analysis. J Urol 2016;196:989-99. [Crossref] [PubMed]
- Tomaszewski JJ, Uzzo RG, Kutikov A, et al. Assessing the burden of complications after surgery for clinically localized kidney cancer by age and comorbidity status. Urology 2014;83:843-9. [Crossref] [PubMed]
- Colombo JR, Haber GP, Jelovsek JE, et al. Seven years after laparoscopic radical nephrectomy: oncologic and renal functional outcomes. Urology 2008;71:1149-54. [Crossref] [PubMed]
- Wasserman M, Sobel D, Pareek G. Choice of Surgical Options in Kidney Cancer and Surgical Complications. Semin Nephrol 2020;40:42-8. [Crossref] [PubMed]
- Gu L, Ma X, Li H, et al. Comparison of oncologic outcomes between partial and radical nephrectomy for localized renal cell carcinoma: A systematic review and meta-analysis. Surg Oncol 2016;25:385-93. [Crossref] [PubMed]
- Steinestel J, Steffens S, Steinestel K, et al. Positive surgical margins in nephron-sparing surgery: Risk factors and therapeutic consequences. World J Surg Oncol 2014;12:252. [Crossref] [PubMed]
- Kryvenko ON. Positive Surgical Margins Increase Risk of Recurrence after Partial Nephrectomy for High Risk Renal Tumors. Shah PH, Moreira DM, Okhunov Z, Patel VR, Chopra S, Razmaria AA, Alom M, George AK, Yaskiv O, Schwartz MJ, Desai M, Vira MA, Richstone L, Landman J, Shalhav AL, Gill I, Kavoussi LR. J Urol. 2016 Aug;196(2):327-34. Urol Oncol 2017;35:449-50. [Crossref] [PubMed]
- Petros FG, Metcalfe MJ, Yu KJ, et al. Oncologic outcomes of patients with positive surgical margin after partial nephrectomy: a 25-year single institution experience. World J Urol 2018;36:1093-101. [Crossref] [PubMed]
- Schiavina R, Mari A, Bianchi L, et al. Predicting positive surgical margins in partial nephrectomy: A prospective multicentre observational study (the RECORd 2 project). Eur J Surg Oncol 2020;46:1353-9. [Crossref] [PubMed]
- Maurice MJ, Zhu H, Kim SP, et al. Reexamining the Association Between Positive Surgical Margins and Survival After Partial Nephrectomy in a Large American Cohort. J Endourol 2016;30:698-703. [Crossref] [PubMed]
- Richstone L, Scherr DS, Reuter VR, et al. Multifocal renal cortical tumors: frequency, associated clinicopathological features and impact on survival. J Urol 2004;171:615-20. [Crossref] [PubMed]
- Tsivian M, Packiam VT, Thompson RH. Tumor Enucleation is Appropriate During Partial Nephrectomy: Against. Eur Urol Focus 2019;5:925-6. [Crossref] [PubMed]
- Siracusano S, Novara G, Antonelli A, et al. Prognostic role of tumour multifocality in renal cell carcinoma. BJU Int 2012;110:E443-8. [Crossref] [PubMed]
- Gupta GN, Peterson J, Thakore KN, et al. Oncological Outcomes of Partial Nephrectomy for Multifocal Renal Cell Carcinoma Greater Than 4 cm. J Urol 2010;184:59-63. [Crossref] [PubMed]
- Laydner H, Autorino R, Spana G, et al. Robot-assisted partial nephrectomy for sporadic ipsilateral multifocal renal tumours. BJU Int 2012;109:274-80. [Crossref] [PubMed]
- Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic Nephrectomy: Initial Case Report. J Urol 2017;197:S182-6. [Crossref] [PubMed]
- Dunn MD, Portis AJ, Shalhav AL, et al. Laparoscopic versus open radical nephrectomy: A 9-year experience. J Urol 2000;164:1153-9. [Crossref] [PubMed]
- Golombos DM, Chughtai B, Trinh QD, et al. Minimally invasive vs open nephrectomy in the modern era: does approach matter? World J Urol 2017;35:1557-68. [Crossref] [PubMed]
- Jeong IG, Khandwala YS, Kim JH, et al. Association of robotic-assisted vs laparoscopic radical nephrectomy with perioperative outcomes and health care costs, 2003 to 2015. JAMA 2017;318:1561-8. [Crossref] [PubMed]
- Golombos DM, Chughtai B, Trinh QD, et al. Adoption of technology and its impact on nephrectomy outcomes, a U.S. population-based analysis (2008-2012) J Endourol 2017;31:91-9. [Crossref] [PubMed]
- Asimakopoulos AD, Miano R, Annino F, et al. Robotic radical nephrectomy for renal cell carcinoma: a systematic review. BMC Urol 2014;14:75. [Crossref] [PubMed]
- Helmers MR, Ball MW, Gorin MA, et al. Robotic versus laparoscopic radical nephrectomy: Comparative analysis and cost considerations. Can J Urol. 2016;23:8435-40. [PubMed]
- Hartman M, Martin AB, Benson J, et al. National Health Care Spending In 2018: Growth Driven By Accelerations In Medicare And Private Insurance Spending. Health Aff (Millwood) 2020;39:8-17. [Crossref] [PubMed]
- Gettman MT, Blute ML, Chow GK, et al. Robotic-assisted laparoscopic partial nephrectomy: Technique and initial clinical experience with daVinci robotic system. Urology 2004;64:914-8. [Crossref] [PubMed]
- Phung MC, Lee BR. Recent advancements of robotic surgery for kidney cancer. Asian J Endosc Surg 2018;11:300-7. [Crossref] [PubMed]
- Banegas MP, Harlan LC, Mann B, et al. Toward greater adoption of minimally invasive and nephron-sparing surgical techniques for renal cell cancer in the United States. Urol Oncol 2016;34:433.e9-433.e17. [Crossref] [PubMed]
- Patel HD, Mullins JK, Pierorazio PM, et al. Trends in renal surgery: Robotic technology is associated with increased use of partial nephrectomy. J Urol 2013;189:1229-35. [Crossref] [PubMed]
- Alameddine M, Koru-Sengul T, Moore KJ, et al. Trends in Utilization of Robotic and Open Partial Nephrectomy for Management of cT1 Renal Masses. Eur Urol Focus 2019;5:482-7. [Crossref] [PubMed]
- Cacciamani GE, Medina LG, Gill T, et al. Impact of Surgical Factors on Robotic Partial Nephrectomy Outcomes: Comprehensive Systematic Review and Meta-Analysis. J Urol 2018;200:258-74. [Crossref] [PubMed]
- Minervini A, Carini M. Tumor Enucleation Is Appropriate During Partial Nephrectomy. Eur Urol Focus 2019;5:923-4. [Crossref] [PubMed]
- García AG, León TG. Simple Enucleation for Renal Tumors: Indications, Techniques, and Results. Curr Urol Rep 2016;17:7. [Crossref] [PubMed]
- Serni S, Vittori G, Frizzi J, et al. Simple enucleation for the treatment of highly complex renal tumors: Perioperative, functional and oncological results. Eur J Surg Oncol 2015;41:934-40. [Crossref] [PubMed]
- Minervini A, Carini M, Uzzo RG, et al. Standardized reporting of resection technique during nephron-sparing surgery: The surface-intermediate-base margin score. Eur Urol 2014;66:803-5. [Crossref] [PubMed]
- Minervini A, Campi R, Kutikov A, et al. Histopathological validation of the surface-intermediate-base margin score for standardized reporting of resection technique during nephron sparing surgery. J Urol 2015;194:916-22. [Crossref] [PubMed]
- Ficarra V, Palumbo V, Kungulli A, et al. Re: Andrea Minervini, Marco Carini, Robert G. Uzzo, Riccardo Campi, Marc C. Smaldone, Alexander Kutikov. Standardized Reporting of Resection Technique During Nephron-Sparing Surgery: The Surface-Intermediate-Base Margin Score. Eur Urol 2014;66:803-5. Eur Urol 2015;67:e45-7. [Crossref] [PubMed]
- Minervini A, Campi R, Sessa F, et al. Positive surgical margins and local recurrence after simple enucleation and standard partial nephrectomy for malignant renal tumors: systematic review of the literature and meta-analysis of prevalence. Minerva Urol Nefrol 2017;69:523-38. [PubMed]
- Xu C, Lin C, Xu Z, et al. Tumor enucleation vs. Partial nephrectomy for T1 renal cell carcinoma: A systematic review and meta-analysis. Front Oncol 2019;9:473. [Crossref] [PubMed]
- Calaway AC, Gondim DD, Flack CK, et al. Anatomic comparison of traditional and enucleation partial nephrectomy specimens. Urol Oncol 2017;35:221-6. [Crossref] [PubMed]
- Lane BR, Samplaski MK, Herts BR, et al. Renal Mass Biopsy-A Renaissance? J Urol 2008;179:20-7. [Crossref] [PubMed]
- Kutikov A, Smaldone MC, Uzzo RG, et al. Renal Mass Biopsy: Always, Sometimes, or Never? Eur Urol 2016;70:403-6. [Crossref] [PubMed]
- Marconi L, Dabestani S, Lam TB, et al. Systematic Review and Meta-analysis of Diagnostic Accuracy of Percutaneous Renal Tumour Biopsy. Eur Urol 2016;69:660-73. [Crossref] [PubMed]
- Ball MW, Bezerra SM, Gorin MA, et al. Grade Heterogeneity in Small Renal Masses: Potential Implications for Renal Mass Biopsy. J Urol 2015;193:36-40. [Crossref] [PubMed]
Cite this article as: Morrison JC, Launer BM, Barqawi ZA, Kim SP. Surgical management of the localized renal mass: risk and benefit trade-offs and surgical approach considerations. AME Med J 2021;6:15.

