
Pulmonary tuberculosis with venous thromboembolism as an unusual presentation of occult pancreatic cancer: a case report
Introduction
Cancer is a well-known established risk factor for thromboembolic events and tuberculosis reactivation due to the immunocompromised condition. However, the simultaneous occurrence of tuberculosis and venous thromboembolism in malignancy is rare. In this case report, we discussed clinical manifestations, investigations and treatment in a patient with pancreatic cancer presented initially with pulmonary tuberculosis (PTB) and unresolved venous thromboembolism. To our best knowledge, this is the first reported case of PTB with venous thromboembolism in pancreatic cancer in our region. We present the following article in accordance with the CARE reporting checklist (available at https://amj.amegroups.com/article/view/10.21037/amj-20-114/rc).
Case presentation
A 65-year-old gentleman with underlying diabetes mellitus, ischemic heart disease and smear positive PTB presented with shortness of breath and left lower limb swelling for 1-week duration. One month ago, patient was newly diagnosed with smear positive PTB with the presentation of chronic cough and loss of weight. His chest radiograph showed consolidation and cavitation in the right upper lobe (Figure 1) with positive sputum for acid fast bacilli (AFB). He was then commenced on standard anti-tuberculous medications.
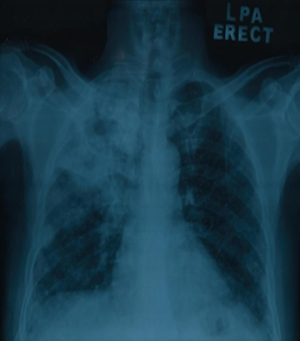
The past medical history included diabetes mellitus and ischemic heart disease. He had no history of smoking and did not drink alcohol. He was a retired general practitioner in a local clinic. His medications prior to the diagnosis included Mixtard insulin injection, bisoprolol and aspirin. There was no family history of malignancy, tuberculosis or hypercoagulable disorder.
Upon review, he was alert, conscious and tachypneic. Vital signs were as follows: blood pressure 112/76 mmHg, heart rate 95 beats per minute, temperature 37 ℃, peripheral capillary oxygen saturation 96% on room air. His left lower limb was swollen, erythematous and oedematous. Respiratory examination revealed crepitations in the right upper zone.
He was diagnosed with left lower limb deep vein thrombosis with pulmonary embolism. His ultrasound (USG) doppler of the left lower limb revealed deep venous thrombosis in the left common femoral, superficial femoral and popliteal veins. On the other hand, computed tomography (CT) pulmonary angiography demonstrated a filling defect in the left inferior lobar artery consistent with pulmonary artery thromboembolism.
Treatment for venous thromboembolism was initiated and he was commenced on Rivaroxaban. The anti-tuberculosis treatment was continued. Notable, he had no other risk factors for thromboembolism. Despite proper treatment with standard anti-tuberculous and adequate anticoagulation, he did not show good clinical improvement and continued to deteriorate. Subsequently, he developed ascites and bilateral pleural effusion while in the ward. Contrast-enhanced CT scan of thorax, abdomen and pelvis showed bilateral lungs consolidative and miliary changes with bilateral pleural effusion, and nodular peritoneal thickenings with gross ascites.
Both diagnostic and therapeutic peritoneal and pleural tappings were done. The cytological examination of pleural and peritoneal fluid demonstrated atypical cells with features consistent with metastatic adenocarcinoma. Tumour markers sent showed high titre of carcinoembryonic antigen (CEA) with 324.1 (normal, 0–5) µg/L and markedly raised serum carbohydrate antigen (CA) 19-9 (89,989.7 U/mL). Triphasic pancreatic-protocol CT scan was done and confirmed lesion at body of pancreas highly suggestive of malignancy (Figure 2).
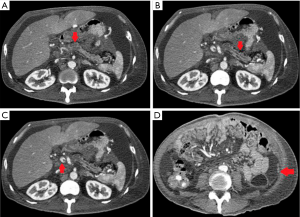
He was diagnosed with pancreatic malignancy after workup. However, biopsy was not proceeded after discussion with family members in view of general ill condition. Palliative care was offered. He succumbed to the illness eventually (Figure 3).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s family member for publication of this case report and any accompanying images.
Discussion
Pancreatic cancer is a tremendously lethal disease. It is characterized by aggressive progression and subtle in initial presentation which make the diagnosis challenging (1). In fact, majority of cases present in advanced state with either locally aggressive or metastatic disease. Multifactorial reasons have been identified. It includes the non-specific symptoms associated with the disease and close proximity of major blood vessels which can be easily invaded by the tumour. Although certain risk factors have been identified, the exact aetiologies of pancreatic cancer are still remained unknown. Numerous studies about occult malignancy in patients with idiopathic deep vein thrombosis have advocated pancreatic cancer among the most common causes identified (2). Several recent studies have shown that the incidence of venous thromboembolism is highest in patients presenting with metastatic cancer, particularly pancreatic cancer and carry poorer prognosis (3).
The relationship between thrombosis and cancer was initially introduced by Armand trousseau in 1865. Multiple form of thrombotic complications in malignancy have been found. It varies from arterial, venous thromboembolism to disseminated intravascular coagulation (4,5). It is well known that pancreatic cancer has a distinct ability to create a hypercoagulable state which subsequently may lead to clinically apparent thrombotic event. The risk factors that have been identified in cancer patients include surgery, immobility, certain comorbidities, tumour stage and histology, the presence of indwelling central venous catheters and chemotherapy (6). The pathophysiology of thromboembolic event in cancer patients is still remained unclear despite the long-recognised association between malignancy and thromboembolic event (7). However, new perceptions into the biological mechanisms responsible for hypercoagulability state in pancreatic cancer have been reported in the last decade, potentially uncover novel therapeutic options (8,9).
Pancreatic cancer expresses peculiarly high level of tissue factor in tumour tissue as well as release of tumour-derived microvesicles. This might lead to activation of extrinsic and intrinsic pathways, promoting platelet activation and adhesion, as well as release of neutrophil extracellular traps from leucocytes, and thus promoting distal thrombosis (10,11). Furthermore, other coagulation pathways probably contribute to these processes, such as those that involve heparinase (12), podoplanin (13,14) and hypofibrinolysis. On the other hand, pancreatic tumour cells and activated platelets can release plasminogen activator inhibitors-1 as a potent inhibitor of fibrinolysis (15,16). In addition to the cancer cell population, pancreatic cancer plays a pivotal role in dynamic milieu of cellular and acellular elements. It includes fibroinflammatory stroma, extracellular matrix and infiltrating immune cells. Thus, postulating that procoagulant character of the cancer cells itself together with the procoagulant properties of the microenvironment that eventually lead to hypercoagulable state in pancreatic cancer (17).
Venous thromboembolic events in tuberculosis is a rare occurrence, but few cases have been reported in literature (18). All the three components of Virchow’s triad, i.e., hypercoagulability, venous stasis, and endothelial dysfunction, can play a role in the pathogenesis of the venous thromboembolism associated with tuberculosis. There are several mechanisms in tuberculosis that can produce a hypercoagulable state that promote thromboembolic complications. There are several studies that have shown increased level of plasma fibrinogen, impaired fibrinolysis along with reduction in antithrombin III, protein C, and reactive thrombocytosis resulting in a hypercoagulable state in which venous thromboembolism can occur in PTB (19,20). However, clinical deterioration with the thrombosis in tuberculosis patient despite proper management alarmed us for the extensive workup of malignancy.
Although tuberculosis and cancer are very common diseases, there has been little attention to the mechanism and clinical implications of their co-existence. The association between tuberculosis and cancer has rarely been reported. Of note, malignancies and the designed therapies for their management seem to create the suitable environment for either the reactivation of a latent mycobacterial infection or, more rarely, for the acquisition of a primary mycobacterial infection. Immunosuppression, principally depression of the T-cell defense mechanisms, is proven to be associated with mycobacterial infections (21). In short, there is paucity of study and analysis regarding the mechanism of tuberculosis in pancreatic cancer. Its association with tuberculosis is a rare occurrence, and very few cases have been reported in literature. To our best knowledge, there was no previous review for the possible link between venous thromboembolism, tuberculosis and pancreatic cancer. Nevertheless, pancreatic cancer has a complex molecular landscape with high potential for future discovery.
The initial diagnosis of PTB in our case masked the diagnosis of pancreatic cancer, in which they shared some of the common constitutional symptoms. Diagnostic challenges arising from the multi-faceted and overlapping presentations of these two disorders. It is well known that the risk of tuberculosis increased in patients with malignant disorders, secondary to immune dysfunction. Thus, tuberculosis and malignancy may co-exist in some cases. The similarities in the clinical and radiological presentations between tuberculosis and malignancy might mislead initial diagnosis and intensify the complexity (22). The limitation in our case report was the biopsy test was not performed. The diagnosis was made based on the triphasic pancreatic protocol CT scan and extreme high titre of CA 19-9.
CA 19-9 was the most well studied and used tumour biomarker for assisting in diagnosis of pancreatic cancer. Furthermore, CEA is likely the second most frequently used biomarker for pancreatic cancer. Nevertheless, neither biomarker owns the clinical accuracy desirable for screening of asymptomatic populations (23). Therefore CA 19-9 and CEA are advisable to be used in conjunction with imaging modality for directing diagnostic as well as treatment decisions in patients with suspected pancreatic cancer.
Pancreatic cancer is one of the most lethal malignancies. Thus, its early detection is crucial. It highlights the need for being more vigilant and a high index of suspicion in patients with unusual or unexplained symptoms.
Conclusions
PTB with venous thromboembolism is a rare mode of presentation for occult pancreatic cancer. Utilisation of CA 19-9 and CEA in the clinically relevant context can be helpful in diagnosis of pancreatic cancer. Furthermore, clinicians need to be aware of the protean manifestations of tuberculosis and cancer thus maintain scepticism for simultaneous or misleading presentations. Further studies regarding cancer associated thrombosis with tuberculosis are warranted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://amj.amegroups.com/article/view/10.21037/amj-20-114/rc
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-20-114/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-20-114/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s family member for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Postier RG. The challenge of pancreatic cancer. Am J Surg 2003;186:579-82. [Crossref] [PubMed]
- Shaib W, Deng Y, Zilterman D, et al. Assessing risk and mortality of venous thromboembolism in pancreatic cancer patients. Anticancer Res 2010;30:4261-4. [PubMed]
- Sproul EE. Carcinoma and venous thrombosis: the frequency of association of carcinoma in the body or tail of the pancreas with multiple venous thrombosis. Am J Cancer 1938;34:566-73.
- Levi M. Cancer-related coagulopathies. Thromb Res 2014;133:S70-5. [Crossref] [PubMed]
- Eichinger S. Cancer associated thrombosis: risk factors and outcomes. Thromb Res 2016;140:S12-7. [Crossref] [PubMed]
- Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: Burden, mechanisms, and management. Thromb Haemost 2017;117:219-30. [Crossref] [PubMed]
- Falanga A, Marchetti M, Russo L. The mechanisms of cancer-associated thrombosis. Thromb Res 2015;135:S8-11. [Crossref] [PubMed]
- Abdol Razak N, Elaskalani O, Metharom P. Pancreatic cancer-induced neutrophil extracellular traps: a potential contributor to cancer-associated thrombosis. Int J Mol Sci 2017;18:487. [Crossref] [PubMed]
- Stark K, Schubert I, Joshi U, et al. Distinct pathogenesis of pancreatic cancer microvesicle-associated venous thrombosis identifies new antithrombotic targets in vivo. Arterioscler Thromb Vasc Biol 2018;38:772-86. [Crossref] [PubMed]
- van den Berg YW, Osanto S, Reitsma PH, et al. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood 2012;119:924-32. [Crossref] [PubMed]
- Khorana AA, Ahrendt SA, Ryan CK, et al. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res 2007;13:2870-5. [Crossref] [PubMed]
- Nadir Y, Brenner B. Heparanase procoagulant activity in cancer progression. Thromb Res 2016;140:S44-8. [Crossref] [PubMed]
- Krishnan H, Rayes J, Miyashita T, et al. Podoplanin: an emerging cancer biomarker and therapeutic target. Cancer Sci 2018;109:1292-9. [Crossref] [PubMed]
- Hirayama K, Kono H, Nakata Y, et al. Expression of podoplanin in stromal fibroblasts plays a pivotal role in the prognosis of patients with pancreatic cancer. Surg Today 2018;48:110-8. [Crossref] [PubMed]
- Chen N, Ren M, Li R, et al. Bevacizumab promotes venous thromboembolism through the induction of PAI-1 in a mouse xenograft model of human lung carcinoma. Mol Cancer 2015;14:140. [Crossref] [PubMed]
- Westrick RJ, Eitzman DT. Plasminogen activator inhibitor-1 in vascular thrombosis. Curr Drug Targets 2007;8:966-1002. [Crossref] [PubMed]
- Campello E, Ilich A, Simioni P, et al. The relationship between pancreatic cancer and hypercoagulability: a comprehensive review on epidemiological and biological issues. Br J Cancer 2019;121:359-71. [Crossref] [PubMed]
- El Fekih L, Oueslati I, Hassene H, et al. Association deep veinous thrombosis with pulmonary tuberculosis. Tunis Med 2009;87:328-9. [PubMed]
- Lang IM, Mackman N, Kriett JM, et al. Prothrombotic activation of pulmonary arterial endothelial cells in a patient with tuberculosis. Hum Pathol 1996;27:423-7. [Crossref] [PubMed]
- Robson SC, White NW, Aronson I, et al. Acute-phase response and the hypercoagulable state in pulmonary tuberculosis. Br J Haematol 1996;93:943-9. [Crossref] [PubMed]
- Karnak D, Kayacan O, Beder S. Reactivation of pulmonary tuberculosis in malignancy. Tumori 2002;88:251-4. [Crossref] [PubMed]
- Falagas ME, Kouranos VD, Athanassa Z, et al. Tuberculosis and malignancy. QJM 2010;103:461-87. [Crossref] [PubMed]
- Kim JE, Lee KT, Lee JK, et al. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol 2004;19:182-6. [Crossref] [PubMed]
Cite this article as: Tan YY, Shuib S, Othman A, Md Yusof MY. Pulmonary tuberculosis with venous thromboembolism as an unusual presentation of occult pancreatic cancer: a case report. AME Med J 2021;6:21.


