
Contemporary review on the management of oral anticoagulation and anti-platelet therapies in patients undergoing percutaneous coronary intervention with concurrent atrial fibrillation
Introduction
With increasing complexities in percutaneous coronary intervention (PCI) and rising prevalence of atrial fibrillation (AF), the need to utilize anticoagulation and dual antiplatelet therapy (DAPT) is frequently encountered in clinical practice, and presents unique challenges in balancing thrombotic risk with bleeding risk. For patients with AF undergoing PCI, the need to have both antiplatelet therapy and anticoagulation is influenced by differing mechanisms of thromboembolism. In AF, thrombosis is predominantly fibrin driven, and in coronary artery disease (CAD), thrombosis is predominantly platelet driven (1). Combination anticoagulation using warfarin, a vitamin K antagonist (VKA), or direct acting oral anticoagulants (DOAC) with antiplatelet therapy has been studied in several contemporary trials. In the United States, warfarin was the only oral anti-coagulant (OAC) available up until 2010, when the Food and Drug Administration (FDA) approved the first DOAC, dabigatran, for prevention of stroke in non-valvular AF (2). Since then, three other DOACs have been approved by the FDA for the same indication: rivaroxaban (3), apixaban (4) and edoxaban (5). Due to reported lower rates of bleeding in the original trials that led to the initial approval of these agents, investigators have sought to evaluate the use of DOACs for patients with AF undergoing PCI, who also require DAPT.
There are 5 major trials that have provided data regarding the utilization of warfarin plus DAPT which is considered standard “triple therapy” or DOAC dual therapy with P2Y12. The first study was WOEST (Use of Clopidogrel with or without Aspirin in Patients Taking Oral Anticoagulant Therapy and undergoing PCI: An open-label, randomised, controlled trial) (6), the second was PIONEER-AF PCI (Prevention of Bleeding in Patients with AF undergoing PCI) (7), the third was RE-DUAL (Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation) (8), the fourth was AUGUSTUS (Antithrombotic Therapy After Acute Coronary Syndrome or PCI in AF) (9), and the most recent trial, ENTRUST AF-PCI (Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation) (10).
The difficulty in balancing bleeding risk with the risk of thromboembolic events requires clinicians to be aware of the benefits and risks of the different therapeutic strategies. Here, we provide a historical review of DAPT in PCI and anticoagulation in AF, as well as provide a contemporary review of the currently available literature regarding triple therapy involving DAPT plus warfarin and dual therapy with DOAC plus a P2Y12. A case description is used to illustrate a difficult clinical scenario, and using this as a basis, we review the contemporary primary literature, and present proposed management algorithms.
Case summary
A 75-year-old patient with AF, severe ischemic cardiomyopathy (left ventricular ejection fraction: 17%) and significant multiple vessel CAD (50% distal left main stenosis, 80% proximal left anterior descending stenosis, 99% proximal left circumflex artery stenosis, and chronic total occlusion of the right coronary artery, with left to right collaterals) and peripheral artery disease (PAD) was admitted to evaluate them for CABG vs. high-risk PCI for their multi-vessel CAD. The patient was deemed not to be a candidate for coronary artery bypass surgery (CABG), due to lack of suitable conduits for surgical revascularization in the context of severe PAD. The patient’s CHA2DS2VASc score of 4 qualified them for anticoagulation. Due to the severity of the CAD, and co-morbidities, the patient was planned to undergo high-risk PCI, under TandemHeart™ (TandemLife Inc, LivaNova, London, UK) support.
The need for anti-platelet therapy following PCI
PCI is performed most commonly with drug eluting stents (DES) and less commonly with bare metal stents (BMS). Antithrombotic therapy has been recognized as necessary to prevent the high rates of stent thrombosis (ST) after stent placement (11). This led to the development of P2Y12 inhibitors, which have been shown to reduce the rates of late ST (11). The first of the P2Y12 inhibitors approved were the oral thienopyridines, clopidogrel (12) and prasugrel (13). Both agents inhibit platelet aggregation by irreversibly binding to the P2Y12 receptor site on platelets, rendering those platelets permanently unable to be activated until the end of the platelet’s lifespan (12,13). A third P2Y12 agent, Ticagrelor (14), was developed as a non-thienopyridine, which is not a prodrug and reversibly inhibits the P2Y12 receptor on platelets (14).
The 2014 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline for the Management of Patients with Non ST Elevation Acute Coronary Syndromes (15), the 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With CAD (16) and the 2020 European Society of Cardiology (ESC) Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation (17) give a Class I recommendation of at least 12 months of DAPT which includes a P2Y12 inhibitor with clopidogrel, prasugrel or ticagrelor as options plus low dose aspirin after DES implantation for ACS (16). These recommendations are based on the results of several trials including CURE (Effects of Clopidogrel in Addition to Aspirin in Patients with Acute Coronary Syndromes without ST-Segment Elevation) (18), COMMIT (Addition of Clopidogrel to Aspirin in 45,852 Patients with Acute Myocardial Infarction; Randomized Placebo-Controlled Trial) (19), PLATO (Ticagrelor versus Clopidogrel in Patients With Acute Coronary Syndromes) (20) and TRITON-TIMI 38 (Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes) (21).
In 2001, the CURE trial (18) provided the initial evidence for initiation of DAPT when the investigators compared 12,562 non-ST elevation myocardial infarction (NSTEMI) patients receiving DAPT with clopidogrel 300 mg × 1 load followed by clopidogrel 75 mg daily for 3–12 months plus aspirin 75–325 mg daily (n=6,259) versus those who received aspirin 75–325 mg alone (n=6,303) (18). The results of the study proved that DAPT with clopidogrel was superior to aspirin alone in reducing the relative risk (RR) of the composite outcome of cardiovascular mortality, incidence of nonfatal MI or stroke (9.3% vs. 11.4%, RR=0.8 P<0.001), but at a cost of an increase in the rate of bleeding in patients with NSTEMI (8.5% vs. 5.0%, RR=1.69, P<0.001) (18). However, since STEMI (ST-elevation myocardial infarction) patients were excluded from this trial, a gap in knowledge was left for those who presented with ST-elevation.
To close that knowledge gap, ST-Elevation myocardial infarction (STEMI) patients were subsequently included in 2005 in the COMMIT (19) trial which randomized 45,852 patients to clopidogrel 75 mg daily (n=22,961) versus placebo added to aspirin in 162 mg daily (n=22,891) and established the use of clopidogrel in patients with STEMI (19). Clopidogrel reduced death compared to placebo [7.5% vs. 8.1%, odds ratio (OR) =0.93, P=0.03] and a composite of death, reinfarction or stroke (9.2% vs. 10.1%, OR =0.91, P=0.002) (19). This trial bridged the gap between NSTEMI and STEMI patients for using DAPT in patients presenting with ACS. CURE (18) and COMMIT (19) proved that clopidogrel was effective.
Subsequently, prasugrel was developed as a more potent P2Y12 inhibitor that produces higher levels of platelet inhibition than clopidogrel (22,23). Prasugrel was investigated in 2007 in the TRITON-TIMI 38 (21) trial that randomized 13,608 patients with ACS to prasugrel 60 mg loading dose followed by prasugrel 10 mg daily (n=6,813) versus clopidogrel 300 mg loading dose followed by clopidogrel 75 mg daily (n=6,795) The trial revealed prasugrel reduced the combined outcome of cardiovascular mortality, nonfatal MI or nonfatal cerebrovascular accident (CVA) [9.9% vs. 12.1%; hazard ratio (HR)=0.81, P<0.001] but with an increase in Non-CABG-related TIMI major bleeding events (2.4% vs. 1.8%, HR=1.32, P=0.03) (21).
Although clopidogrel and prasugrel were shown to be effective, they both have a pharmacokinetic disadvantage of needing to be metabolized into an active form and also a pharmacodynamic disadvantage of binding irreversibly to the P2Y12 receptor site (12,13). Awaiting metabolism to take place slows onset and irreversible binding to P2Y12 receptors permanently impairs platelet function. Furthermore, although prasugrel is included in the 2014 ACC/AHA Non-ST-Elevation Acute Coronary Syndromes guidelines (15) and 2016 ACC/AHA focused updated on duration of DAPT after ACS guidelines for use in PCI after ACS (16), it is not often used in clinical practice. Prasugrel showed no net benefit in a subset of patients in TRITON TIMI 38 (21) that were ≥75 years of age, patients weighing <60 kilograms or in patients who have had a history of transient ischemic attack/stroke (16) due to the increased rates of bleeding in those groups. In fact, for patients with previous transient ischemic attack/stroke a class III (harm) recommendation was established against the use of prasugrel due to increased risk of intracranial hemorrhage (21). The 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation (17) recommend prasugrel over ticagrelor and clopidogrel in patients who will receive PCI in the setting of non-ST elevation ACS partially based on the results of the ISAR-REACT 5 trial. Additionally, the 2020 ESC ACS NSTEMI guidelines recommend a reduced dose of prasugrel 5 mg for patients who are ≥75 years of age and patients weighing <60 kilograms, which was the recommended dosing strategy in those patients in the ISAR-REACT trial. ACC/AHA guidelines have not been updated to include the ISAR-REACT 5 data.
Ticagrelor was soon developed as a P2Y12 inhibitor which is not a prodrug, and binds reversibly to an allosteric site on platelets (24), circumventing the disadvantages of clopidogrel and prasugrel. Additionally, ticagrelor is a more potent platelet inhibitor that has an 88% inhibition of platelet aggregation (IPA) compared to clopidogrel that has a 66% IPA after a loading dose of each drug (25). In 2009, the PLATO trial randomized 18,624 patients with ACS to ticagrelor 180 mg loading dose followed by 90 mg twice daily (n=5,123) versus clopidogrel 300-600 mg loading dose followed by 75 mg daily (n=5,128). PLATO revealed ticagrelor reduced the composite outcome of vascular mortality, myocardial infarction or CVA (9.8% vs. 11.7%, HR=0.84, P<0.001), with a small increase in TIMI (thrombolysis in myocardial infarction) major bleeding (7.9% vs. 7.7%, HR=1.03, P=0.57) (20).
In 2019, the ISAR-REACT 5 (Ticagrelor or Prasugrel in Patients with Acute Coronary Syndrome) (26) compared ticagrelor to prasugrel in a trial which randomized 4,018 patients with ACS to ticagrelor 180 mg loading dose followed by 90 mg twice daily (n=2,012) versus prasugrel loading dose of 60 mg followed by 10 mg daily in (n=2,006) or 5 mg daily maintenance dose in patients who were ≥75 years of age or patients weighing <60 kilograms (n=2,006) (26). It revealed a lower incidence of the primary composite outcome of death, nonfatal MI or stroke (9.3% vs. 6.9%, HR 1.36, P<0.01) and a lower incidence of BARC (Bleeding Academic Research Consortium) major bleeding in the prasugrel arm (5.4% vs. 4.8%, HR=1.12, P=0.46) (26).
The need for anticoagulation in AF
The need for anticoagulation in AF is aimed at prevention of ischemic stroke (27). An AF patients’ risk of developing ischemic stroke is stratified using the CHA2DS2VASc score which allows clinicians to estimate a patient’s 1-year percentage risk of developing ischemic stroke and decide on the utilization of anticoagulation (27). The 2019 ACC/AHA focused update to the 2014 atrial fibrillation guidelines (27) as well as the 2020 ESC atrial fibrillation guidelines (28) give a class I recommendation to initiating oral anticoagulation for male patients with a CHA2DS2VASc score of ≥2, and female patients with a CHA2DS2VASc score of ≥3 to reduce the risk of stroke or systemic embolism (27,28). Historically, warfarin, targeted to an international normalization ratio (INR) of 2.0 to 3.0, has been the standard of care for oral anticoagulation in patients with AF (29). In 2010, DOACs were introduced as alternatives to warfarin for patients with AF. In our case, the patients CHA2DS2VASc score was 4, which qualified them for oral anticoagulation, and they were managed with DOAC therapy prior to admission.
DOACs promoted several advantages over warfarin, including therapeutic efficacy within hours of administration, no need for routine INR testing and no dietary restrictions for patients (28,30). However, DOAC agents require strict patient adherence to dosing regimens in order to maintain effectiveness (27). The 2019 ACC/AHA/Heart Rhythm Society (HRS) focused update to the 2014 guidelines (27) as well as the 2020 ESC atrial fibrillation guidelines (28) give a class I recommendation to choosing a DOAC such as apixaban, rivaroxaban, dabigatran or edoxaban over warfarin; except in patients with moderate to severe mitral stenosis or those with a mechanical heart valve (27,28). Warfarin is still the preferred agent for patients diagnosed with AF who have a history of moderate-severe mitral stenosis or a mechanical heart valve (27,28) because these patients were excluded from the DOAC trials, and therefore the safety and efficacy are not fully known. Dabigatran was given a class III (harm) recommendation (27) for patients with mechanical valves, as it caused an increased risk of stroke or systemic embolism in the RE-ALIGN (Dabigatran versus Warfarin in Patients with Mechanical Heart Valve) trial (31).
To help balance thrombotic risk with bleeding risk there are several tools used to stratify bleeding risk, including the HAS-BLED score (29), HEMORRHAGES score (29) and ATRIA score (29). HAS-BLED is the most commonly used and estimates the 1-year risk of a patient on warfarin having a major bleeding event (29). HAS-BLED has not been validated in DOAC’s and there is some evidence that it may not be predictive of bleeding risk in patients on DOAC (32). In this case the patient was on DOAC and therefore a HAS-BLED score was not used to stratify bleeding risk.
In 1996, one of the first studies to evaluate the efficacy of OAC in patients with AF was the SPAF-III trial (Stroke Prevention in Atrial Fibrillation III Study). SPAF-III randomized 1,044 patients to either low intensity warfarin therapy including aspirin 325 mg per day plus warfarin dose adjusted to INR =1.2–1.5 (n=521) versus standard warfarin dose, adjusted INR goal of 2.0–3.0 (n=523) for patients with AF to prevent stroke (33). The trial was stopped early due to the significant increase in death, ischemic stroke, and systemic emboli in the low intensity group (1.9% warfarin vs. 7.9% low intensity warfarin + aspirin, P<0.001) (33). SPAF-III solidified the use of dose adjusted warfarin with an INR goal of 2.0–3.0 for patients with AF to reduce the risk of ischemic stroke (33). With the many challenges warfarin posed to patients, it was recognized that patients could benefit from OAC that did not require regular blood tests, dosage adjustments and dietary restrictions, which led to the development of DOACs (4).
In 2009, RE-LY (Dabigatran versus Warfarin in Patients with Atrial Fibrillation) (2) was the first successful clinical trial to compare a DOAC, dabigatran a direct factor IIa inhibitor, versus warfarin in patients with AF for the reduction of stroke risk (2). RE-LY randomized 18,133 patients to receive low dose dabigatran 110 mg twice daily n=6,015, high dose dabigatran 150 mg twice daily (n=6,076) or dose adjusted warfarin (n=6,022), INR goal of 2–3 (2). The trial revealed dabigatran 150 mg daily was superior to warfarin in reducing stroke and systemic embolism (1.11% vs. 1.69%, RR =0.66, P=0.001) (2), and also showed a lower rate of combined major or minor bleeding (18.15% vs. 16.42%, P=0.002) including lower rates of intracranial bleeding (2). These were landmark findings, and opened the door to DOACs being approved for use in AF for the prevention of stroke.
In 2011, The ROCKET-AF (Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation) trial compared rivaroxaban, the first of the oral direct Xa inhibitor DOACs versus warfarin in patients with AF in a non-inferiority study (3). Rivaroxaban has the same advantages of dabigatran over warfarin in not needing routine blood tests or dosage adjustments. Notably rivaroxaban was tested as a once daily dosing regimen which would be potentially more convenient for patients than dabigatran twice daily dosing (3). Rocket-AF randomized 14,264 patients to rivaroxaban 20 mg daily (n=7,131) versus dose adjusted warfarin, INR goal of 2.0–3.0 (n=7,133) (3). The trial revealed that rivaroxaban 20 mg daily was non-inferior to warfarin in reducing the risk of stroke or systemic embolism in the per protocol (PP) (1.7% vs. 2.2%, HR=0.79, P<0.001) and the intention-to-treat (ITT) populations (2.1% vs. 2.4%, HR=0.88, P=0.001), and had lower incidence of intracranial hemorrhage (0.8% vs. 1.2%, HR=0.67, P=0.02) compared to warfarin (3).
In 2011, the ARISTOTLE (Apixaban versus Warfarin in patients with Atrial Fibrillation) trial (34) compared apixaban, the second of the direct Xa inhibitor DOACs versus warfarin in patients with AF. ARISTOTLE randomized 18,201 patients to receive apixaban 5 mg twice daily (n=9,120) or dose adjusted warfarin, INR goal of 2.0–3.0 (n=9,081), in patients with AF (34). The trial revealed that apixaban 5 mg twice daily was superior to dose adjusted warfarin at reducing the risk of stroke or systemic embolism (1.27% vs. 1.6%, HR=0.79, P=0.01) (34) and had lower rates for ISTH (International Society of Thrombosis and Haemostasis) major bleeding (2.13% vs. 3.09%, HR=0.69, P<0.001) (34) including intracranial bleeding (0.33% vs. 0.8%, HR=0.42, P≤0.001) (34).
The data for edoxaban was published in 2013 in the ENGAGE-TIMI 48 (Edoxaban versus Warfarin in Patients with Atrial Fibrillation) trial (5) which randomized 21,105 patients to receive high dose edoxaban 60 mg daily (n=7,035) or low dose edoxaban 30 mg daily (n=7,034) versus dose adjusted warfarin, goal INR 2.0–3.0 (n=7,036) in patients with AF for the prevention of stroke (5). The trial revealed that edoxaban 60 mg daily reduced the risk of stroke or systemic embolism (1.18% vs. 1.5%, HR=0.79, P<0.001 for superiority) and edoxaban 30 mg was non-inferior to warfarin in reducing risk of stroke or systemic embolism (1.61% vs. 1.5%, HR=1.07 P=0.005 for non-inferiority) (5). Both high dose and low dose edoxaban had a lower rate of major bleeding (high dose: 2.75% vs. 3.43%, HR 0.8, P<0.001; low dose: 1.61% vs. 3.43%, P<0.001), including lower rates of intracranial bleeding (5).
All four DOAC agents were found to either be non-inferior or superior to warfarin in prevention of stroke and systemic embolism, and were as associated with reduced rates of bleeding events, in particular intracranial hemorrhage (28). This was a very important finding as intracranial hemorrhage is a serious and significant side effect found in patients on anticoagulation. These four trials solidified DOACs as very good alternatives to warfarin for AF patients. Dabigatran, rivaroxaban and apixaban have all found their way into guidelines and routine clinical practice (28,30). Edoxaban has found its way into guidelines but is not used on a routine basis, due to the manufacturers’ recommendation to avoid utilization of edoxaban in patients with a creatine clearance >95 mL/min due to increased rates of ischemic stroke in such patients in the ENGAGE-TIMI 48 trial (10,35). About 50% of edoxaban is renally eliminated and blood levels of edoxaban are decreased by 40% in patients with a creatinine clearance of >95 mL/min, it is anticipated that patients with better renal function edoxaban will show smaller effect on stroke/systemic embolism reduction in those patients (35).
The need for anticoagulation and anti-platelet therapies in patients with AF undergoing percutaneous coronary intervention
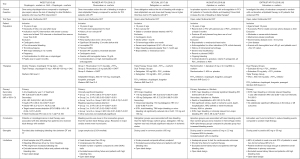
The unique balancing act of managing patients who have AF and require PCI either emergently or electively was recently addressed in the 2020 ESC Atrial fibrillation guidelines (28) with recommendation level grading and in the 2020 ACC Expert Consensus Decision pathway for anticoagulant and antiplatelet therapy in patients with AF or venous thromboembolism (30) (Figure 1). Level of evidence grading was not given in the 2020 ACC Expert Consensus Decision pathway (30) for recommendations made, as it is a consensus statement and not a guideline, reflecting largely the relatively limited evidence base for some of the updates. None of the published guidelines recommend DOAC for triple therapy long-term (27,28,30). Instead, the 2020 ESC atrial fibrillation guidelines (28) give a level I recommendation for either dual therapy including DOAC combined with a P2Y12 agent, preferably clopidogrel and a Level IIa recommendation to consider triple therapy with warfarin as the anticoagulant for those who do not qualify for DOAC and clopidogrel as the preferred P2Y12, (28,30). The 2020 ACC Expert Consensus Decision pathway (30) allows for triple therapy with warfarin as an option for patients who were originally on warfarin prior to PCI (30). However, dual therapy with DOAC combined with a P2Y12 agent is preferred if the patient is eligible for DOAC, owing to their generally lower risk of major fatal and intracranial bleeding compared with warfarin, simplicity, rapid onset of action and lack of need for bridging (30). This is in line with the 2019 ACC/AHA/HRS guidelines (27) and in agreement with the 2020 ESC atrial fibrillation guidelines (28), which gives reduced dose rivaroxaban and normal dose dabigatran in combination with clopidogrel a level II recommendation as therapeutic options to reduce the risk of bleeding compared to triple therapy (27). Apixaban is not listed as an agent in the 2019 ACC/AHA/HRS guidelines (27) for double or triple therapy, as the AUGUSTUS trial had not been published prior to the release of the 2019 guidelines (30). However, the 2020 ACC expert consensus decision pathway statement (30) includes apixaban as an option in combination with clopidogrel for dual therapy (30).

The 2020 ACC expert consensus decision pathway allows for low dose aspirin to be continued for the duration of the hospitalization after PCI, but in general should be discontinued upon discharge, or, at 30 days in those patients who are a high thrombotic risk and low bleeding risk (30). Patients who do not qualify for DOAC therapy and will have AC managed with coumadin may continue low dose aspirin along with clopidogrel until the INR is therapeutic (28,30). Patients with ACS who have a low risk for stent thrombosis or have concerns about bleeding risk, ESC Atrial Fibrillation guidelines (28) give a level I recommendation to early cessation of aspirin (≤1 week) and continuation of dual therapy with oral anticoagulation (preferably DOAC) and a P2Y12 inhibitor (preferably clopidogrel for up to 12 months) (28). For AF patients presenting with ACS where stent thrombosis outweighs bleeding risk, a level II recommendation is given for continuation of aspirin for >1 week but ≤1 month (28).
When choosing a P2Y12 agent, the 2020 ACC expert consensus statement and the 2020 ESC guidelines are clear that clopidogrel is the agent of choice due to a higher risk of bleeding with both prasugrel and ticagrelor (30). The 2019 ACC/AHA/HRS guidelines only mention the use of ticagrelor in the context of double therapy with dose adjusted warfarin (27). Clopidogrel was the agent used in >90% of patients in both the RE-DUAL PCI trial (8) and AUGUSTUS trial (9), ticagrelor and prasugrel were represented, albeit in small numbers (8,9). Triple therapy with prasugrel was evaluated in a small study titled “Triple Therapy with Aspirin, Prasugrel and Vitamin K Antagonist in Patients with Drug Eluting Stent Implantation and an Indication for Oral Anticoagulation” (37) that randomized 377 patients to evaluate the incidence of TIMI major and minor bleeding at 6 months (clopidogrel n=356, prasugrel n=21). Prasugrel had a 4-fold higher rate of TIMI major bleeding (28.6% vs. 6.7%, HR=4.6, P≤0.001) (37). Based on these data, prasugrel is not recommended to be used in any patients needing double therapy or triple therapy (28,30).
There have been 5 large contemporary clinical trials that have investigated combined antiplatelet and anticoagulation regimens (Figure 2). In 2013, the WOEST trial (6) was the first to evaluate triple therapy using warfarin and P2Y12 inhibitors (6). WOEST randomized 573 patients (double therapy n=279, triple therapy n=284) to receive clopidogrel 75 mg daily plus dose adjusted warfarin with an INR target of 2.0 versus clopidogrel 75 mg daily plus aspirin 80–100 mg daily and dose adjusted warfarin with an INR target of 2.0 (6). The investigators sought to compare the rates of any bleeding between dual therapy with warfarin or triple therapy group. The trial revealed a lower incidence of bleeding events in the double therapy group (19.4% vs. 44.4%, HR=0.36, P<0.0001) (6). There was a numerically higher, but non-significant incidence of need for target-vessel revascularization in the double therapy group vs. the triple therapy group (7.2% vs. 6.7%, HR=1.05, P=0.876) (6). Of note, the trial was not powered to detect a difference for target vessel revascularization. WOEST led to the 2014 ACC/AHA/HRS atrial fibrillation guidelines giving an option to choose clopidogrel and a VKA without aspirin as a consideration for patients with AF in the setting of ACS (29).
The first trial to test dual therapy with DOACs was published in 2014, when PIONEER-AF randomized 2,124 patients to receive low dose rivaroxaban 15 mg plus P2Y12 (n=709), very-low-dose rivaroxaban 2.5 mg plus DAPT (n=709) or dose adjusted warfarin, INR goal of 2.0–3.0, plus DAPT, which was considered standard therapy (n=706) (7). The low dose rivaroxaban group had a lower incidence of the primary safety outcome of clinically significant bleeding than the standard therapy group (16.8% vs. 26.7%, HR=0.59, P=0.001), while maintaining a similar incidence of major adverse cardiovascular events (MACE) and stent thrombosis vs. standard therapy (6.5% in low dose rivaroxaban group vs. 6.0% in standard therapy group, HR=1.08, P=0.75) (7). This trial led to a level IIa recommendation for low dose rivaroxaban plus clopidogrel as a reasonable alternative to standard triple therapy in the 2019 ACC/AHA/HRS guidelines (27).
Dabigatran was the second DOAC to be evaluated in the RE-DUAL study which randomized 2,725 patients to receive low dose dabigatran 10 mg twice daily (n=981), normal dose dabigatran 150 mg twice daily (n=763) or standard triple therapy with adjusted dose warfarin, INR goal of 2.0–3.0 (n=981) (8). Both dabigatran groups had a significantly lower incidence of the primary endpoint of ISTH major bleeding or clinically relevant non-major (CRNM) bleeding (15.4% in low dose group vs. 26.9% in standard therapy group, HR=0.52, P=0.001; 20.2% in standard dose dabigatran group vs. 25.7% in standard therapy group; HR=0.72, P=0.002) (8). However, only standard dose dabigatran dose was found to be non-inferior to standard therapy for the composite efficacy endpoint of thromboembolic events, death or unplanned revascularization (8). This led to a level IIa recommendation in the 2019 ACC/AHA/HRS guidelines for dual therapy with standard dose dabigatran as a reasonable alternative to triple therapy to reduce the risk of bleeding (27).
In 2019, the AUGUSTUS trial randomized 4,614 patients, using a two-by-two factorial trial design comparing patients who received combinations of apixaban or warfarin and aspirin or placebo (9). For patients who received apixaban, a lower incidence of ISTH major bleeding or CRNM bleeding was found (10.5% vs. 14.7%, HR=0.69, P<0.001) and a numerically lower but non-significant rate of death or ischemic event (6.7% vs. 7.1%, HR=0.93, P value is non-significant) versus patients who received warfarin (9). For patients who received aspirin, there was a higher incidence of ISTH major bleeding or CRNM bleeding (16.1% vs. 9.0%, HR=1.89, P<0.001) and a numerically lower incidence of death or ischemic event (6.5% vs. 7.3%, HR=0.89) (9). What is also important is that rates of death or ischemic events were numerically lower with patients who received apixaban versus those who received warfarin (6.7% vs. 7.1% HR 0.93, superiority P=NS) (9). Additionally, in other secondary efficacy outcomes of a composite of death or hospitalization or a composite of death or ischemic events, there was no statistically significant difference in rates events found between patients who received aspirin and those who did not. This led to the recommendation to include apixaban plus clopidogrel as an option for therapy in both the 2020 ACC expert consensus document and the 2020 ESC atrial fibrillation guidelines (28,30).
Most recently in 2020, the ENTRUST-AF trial randomized 1,506 patients to compare edoxaban (n=751) standard dose plus P2Y12 inhibitor (dose of edoxaban could be reduced for patients with creatinine clearance 15–50 mL/min, body weight ≤60 kg or concurrent use of P-glycoprotein inhibitors) versus standard triple therapy with dose adjusted warfarin, INR goal of 2.0–3.0 (n=755) (10). The baseline P2Y12 inhibitor was clopidogrel in each group, but could be changed to prasugrel or ticagrelor at the discretion of the investigator (10). The edoxaban group was noninferior to warfarin group for the primary outcome of ISTH major or CRNM bleeding (17% vs. 20%, HR=0.83, non inferiority P=0.001), but there was a non-significant increase in the composite of cardiovascular death, stroke, systemic embolism, MI or definite stent thrombosis for patients in the edoxaban group (7% vs. 6%, HR=1.06, 95% CI, 0.71–1.69) (10).
Taken together the current available evidence, the various proposed therapeutic management pathways for patients with AF undergoing AF, requiring anticoagulants and anti-platelet therapies are shown in Figure 3. Note that the drug dosing in Figure 3 is a general guide only, based on current published data, and should not be relied upon to make clinical decisions for individual patients.
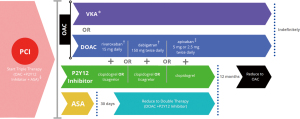
In summary, the risk of stent thrombosis is highest in the early phase after PCI and declines over time and the risk of bleeding with triple therapy increases with duration of therapy (27). Clopidogrel is the P2Y12 agent of choice recommended by both the ACC and ESC (28,30). Long term use of triple therapy with warfarin or any of the DOACs is not preferred in either the 2020 ACC expert consensus or 2020 ESC atrial fibrillation guidelines. However, both documents do allow for low dose aspirin to be given for up to 30 days in patients at high risk for stent thrombosis (28,30) with early cessation, discontinuation within 7 days, recommended for uncomplicated PCI cases (28) (Figure 3). Although DOACs are preferred, for a group of patients where warfarin is used, low dose aspirin can be used until the INR is therapeutic between 2.0 and 2.5 (28,30). For selection of an antiplatelet agent, clopidogrel is the drug of choice recommended by both the 2020 ACC consensus statement and the 2020 ESC guidelines (Table 1). Recommendations for duration of P2Y12 therapy will follow the 2016 ACC/AHA Guideline Focused update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease (16).
Table 1
| Guideline | Year published | Assessment tools (CHA2DS2-VASc, HAS-BLED) | Antiplatelet of choice | Anticoagulant of choice (DOAC/VKA) | Duration of triple therapy before de-escalation | |
|---|---|---|---|---|---|---|
| Condition type | Time | |||||
| American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Rhythm Society (HRS) (27) | 2019 update | High stroke risk: CHA2DS2-VASc risk score of ≥2 High risk of bleeding: HAS-BLED score ≥3 |
Clopidogrel | DOAC or VKA | High stroke risk | 4–6 weeks† |
| European Society of Cardiology (ESC) with European Association for Cardio-Thoracic Surgery (EACTS) (28) | 2020 | High stroke risk: CHA2DS2-VASc risk score of ≥2 High risk of bleeding: HAS-BLED score ≥3 |
Clopidogrel | DOAC | Low thrombosis risk or bleeding risk outweighs thrombosis | ≤1 week |
| High risk of stent thrombosis outweighs bleeding risk | ≤4 weeks | |||||
| American College of Chest Physicians (CHEST) (36) | 2018 | High stroke risk: CHA2DS2-VASc risk score of ≥2 High risk of bleeding: HAS-BLED score ≥3 |
Clopidogrel | DOAC or VKA | Elective PCI and low bleeding risk | 1–3 months |
| High bleeding risk | 1 month | |||||
| Low bleeding risk | 6 months | |||||
†According to a 2020 update from the American College of Cardiology, triple therapy is recommended is to last 30 days at which point low dose aspirin therapy is discontinued. AF, atrial fibrillation; PCI, percutaneous coronary intervention; DOAC, direct acting oral anticoagulant; VKA, vitamin K antagonist.
Case follow-up
Our patient underwent successful complex PCI to the left main artery, left anterior descending artery and left circumflex artery, under TandemHeart™ (TandemLife Inc, LivaNova, London, UK) support. The patient was discharged on aspirin 81 mg daily and clopidogrel 75 mg daily, with the plan to discontinue aspirin after 2 weeks, and initiate apixaban 5 mg twice daily, in addition to the clopidogrel 75 mg daily. Ultimately, the patient will be maintained on dual therapy with clopidogrel and apixaban. The antithrombotic strategy varied slightly from the strategy recommended in the current ACC/AHA guidelines. This reflects the patient’s iron deficiency anemia and hemoglobin drop as an inpatient, and suspected gastrointestinal tract bleeding, although no obvious source of bleeding was found on gastroscopy and colonoscopy. The decision to add anticoagulation with apixaban and stop aspirin at 2 weeks could have been partially influenced by the results of the AUGUSTUS trial, showing separation of the Kaplan-Meier curve at 14 days for the primary outcome of ISTH major or clinically relevant non-major bleeding with a lower bleeding risk without aspirin (9). This case illustrates the complexity of the decision making involved in antithrombotic and antiplatelet therapy management for patients with AF undergoing PCI.
Conclusions and future perspectives
The balance between thrombotic events and bleeding risk will continue to be a challenge for clinicians to navigate in patients undergoing percutaneous coronary intervention with a concomitant diagnosis of AF. Several contemporary clinical trials provide clinically useful data to guide clinicians in decision-making. Major society guidelines have also provided updated recommendations regarding the choice of antithrombotic and antiplatelet therapy regimens in these complex scenarios. Ultimately, the antithrombotic and antiplatelet therapy for each individual patient must be tailored to each patient’s clinical situation, and overall thrombotic and bleeding risks. More prospective multi-center data, including further randomized controlled trials, will provide better guidance for clinicians on the optimal combinations and durations of antithrombotic and antiplatelet therapies for patients with AF undergoing percutaneous coronary intervention.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://amj.amegroups.com/article/view/10.21037/amj-21-9/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-21-9/coif). Dr. Ramone Boyd received an unsolicited Honoraria from Case Western Reserve University on April 14, 2021 for a guest lecture titled “Pharmacologic management of patients with Acute Coronary Syndrome and Stable Ischemic Heart Disease” at the School of Nursing for course NURS 430 Advanced Pharm on April 6, 2021. Dr. Bo Xu serves as an unpaid editorial board member of AME Medical Journal. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). The publication of this manuscript is waived from patient informed consent according to the ethics committee or institutional review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Agarwal N, Mahtta D, Rambarat CA, et al. Optimum Antithrombotic Therapy in Patients Requiring Long-Term Anticoagulation and Undergoing Percutaneous Coronary Intervention. Biomed Res Int 2018;2018:5690640. [Crossref] [PubMed]
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. [Crossref] [PubMed]
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. [Crossref] [PubMed]
- Xu B, Whitbourn R. Novel anticoagulants for non-valvular atrial fibrillation. Heart Lung Circ 2012;21:463-7. [Crossref] [PubMed]
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104. [Crossref] [PubMed]
- Dewilde WJ, Oirbans T, Verheugt FW, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 2013;381:1107-15. [Crossref] [PubMed]
- Gibson CM, Mehran R, Bode C, et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N Engl J Med 2016;375:2423-34. [Crossref] [PubMed]
- Cannon CP, Bhatt DL, Oldgren J, et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N Engl J Med 2017;377:1513-24. [Crossref] [PubMed]
- Lopes RD, Heizer G, Aronson R, et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N Engl J Med 2019;380:1509-24. [Crossref] [PubMed]
- Vranckx P, Valgimigli M, Eckardt L, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet 2019;394:1335-43. [Crossref] [PubMed]
- Reejhsinghani R, Lotfi AS, et al. Prevention of stent thrombosis: challenges and solutions. Vasc Health Risk Manag 2015;11:93-106. [PubMed]
- Plavix (clopidogrel) package insert. Bridgewater, NJ: Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership; May 2019.
- Effient (prasugrel) package insert. Indianapolis, IN: Eli Lilly and Company; November 2020.
- Brilinta (ticagrelor) package insert. In: Pharmaceuticals A, editor. Wilmington, DENovember 2020.
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139-228. [Crossref] [PubMed]
- Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2016;152:1243-75. [Crossref] [PubMed]
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289-367. [Crossref] [PubMed]
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494-502. [Crossref] [PubMed]
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1607-21. [Crossref] [PubMed]
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57. [Crossref] [PubMed]
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15. [Crossref] [PubMed]
- Michelson AD, Frelinger AL 3rd, Braunwald E, et al. Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial. Eur Heart J 2009;30:1753-63. [Crossref] [PubMed]
- Schnorbus B, Daiber A, Jurk K, et al. Effects of clopidogrel vs. prasugrel vs. ticagrelor on endothelial function, inflammatory parameters, and platelet function in patients with acute coronary syndrome undergoing coronary artery stenting: a randomized, blinded, parallel study. Eur Heart J 2020;41:3144-52. [Crossref] [PubMed]
- Angiolillo DJ, Rollini F, Storey RF, et al. International Expert Consensus on Switching Platelet P2Y12 Receptor-Inhibiting Therapies. Circulation 2017;136:1955-75. [Crossref] [PubMed]
- Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation 2009;120:2577-85. [Crossref] [PubMed]
- Schüpke S, Neumann FJ, Menichelli M, et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N Engl J Med 2019;381:1524-34. [Crossref] [PubMed]
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2019;16:e66-93. [Crossref] [PubMed]
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373-498. [Crossref] [PubMed]
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1-76. [Crossref] [PubMed]
- Kumbhani DJ, Cannon CP, Beavers CJ, et al. 2020 ACC Expert Consensus Decision Pathway for Anticoagulant and Antiplatelet Therapy in Patients With Atrial Fibrillation or Venous Thromboembolism Undergoing Percutaneous Coronary Intervention or With Atherosclerotic Cardiovascular Disease: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2021;77:629-58. [Crossref] [PubMed]
- Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013;369:1206-14. [Crossref] [PubMed]
- Tsu LV, Berry A, Wald E, et al. Modified HAS-BLED Score and Risk of Major Bleeding in Patients Receiving Dabigatran and Rivaroxaban: A Retrospective, Case-Control Study. Consult Pharm 2015;30:395-402. [Crossref] [PubMed]
- Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: Stroke Prevention in Atrial Fibrillation III randomised clinical trial. Lancet 1996;348:633-8. [Crossref] [PubMed]
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. [Crossref] [PubMed]
- Savaysa (edoxaban) package insert. Tokyo, Japan: Daiichi Sankyo; April 2020.
- Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest 2018;154:1121-201. [Crossref] [PubMed]
- Sarafoff N, Martischnig A, Wealer J, et al. Triple therapy with aspirin, prasugrel, and vitamin K antagonists in patients with drug-eluting stent implantation and an indication for oral anticoagulation. J Am Coll Cardiol 2013;61:2060-6. [Crossref] [PubMed]
Cite this article as: Boyd RL, Tasseff N, Xu B. Contemporary review on the management of oral anticoagulation and anti-platelet therapies in patients undergoing percutaneous coronary intervention with concurrent atrial fibrillation. AME Med J 2021;6:31.

