
Saturated phosphatidylcholine as dietary additive for colonic mucus: an open label prospective clinical observation trial
Introduction
Phosphatidylcholines (PCs) provide protection in intestinal mucus where they function as hydrophobic barrier against microbiota (1). PC consists of a glycerol molecule, which is esterified with phosphoric acid being linked to choline and two further fatty acids. The fatty acids may be saturated or unsaturated. The degree of saturation of the fatty acids in PC in mucus varies, dependent on the respective mucosa. In intestinal mucus most of the PCs contain at least one unsaturated fatty acid, such as PC 16:0/18:1, PC 16:0/18:2, and PC 18:0/18:1 (2). In contrast, the main PC species in lung alveoli is saturated PC 16:0/16:0, because otherwise its double bounds are prone to oxidation causing free radical injury (3).
The protective function of mucus is of particular relevance in the large intestine due to the exposure with one trillion bacteria per g of stool. In the lung it is known as surfactant and also responsible for gas exchange. The positive polar choline headgroup is bound to mucins, mainly secretory mucin-1 and mucin-2, while the lipophilic fatty acid side chain stretches to the lumen (4). Around the net of mucin bundles the generated hydrophobicity repels luminal bacteria from entering the mucus. As shown for the intestine within this layer microbiota fades and becomes absent in the attached mucus close to the mucosal cell surface (5). Between the bundles an electrolyte rich fluid still allows nutrients to enter for absorption by mucosal cells.
PC is actively transported in the intestine to the apical side of mucosal cells via a transcellular movement across the lateral tight junction barrier driven by a negative electrical potential generated by the cystic fibrosis transmembrane conductance regulator (CFTR) (6,7). Mucus PC originates from lipoproteins within the circulation. Disruption of the tight junction mediated transport in intestine is reported to be causative in ulcerative colitis (7)—a mechanism which most likely is of genetic origin. It is also conceivable that mucus PC depletion can be acquired secondary to inflammation or ischemia of any reason. Accordingly the PC binding sites on secretory mucins remain empty. As a consequence of the low mucus PC content as it was shown in ulcerative colitis (2,8), hydrophobicity is reduced (9) and microbiota can invade causing mucosal inflammation (5). Due to the intrinsic defect of this transport mechanism in ulcerative colitis the lack of PC cannot be compensated from inside the body by systemic application of this phospholipid. Moreover, some colonic bacteria species contain on their surface ectophospholipases, which consume mucus PC for energy generation. In case these strains overgrow due to any circumstances they affect the mucus barrier stability (10).
The fact that mucus PC originates from systemic sources, which is disturbed in ulcerative colitis, it can therapeutically only be targeted by luminal supplementation. Natural PC contains at least 50% unsaturated fatty acid substitutes and is completely absorbed in the upper small intestine after it is cleaved by pancreatic phospholipases to lyso-PC and fatty acids. Therefore, it is hardly available as luminal PC in distal intestine and colon. To enable PC to enter the colon as intact molecule from the intestinal lumen for occupation of the empty binding sites on mucin-2, the strategy is to make it non-accessible to pancreatic phospholipases. An already proven option is the preparation of PC formulations with certain enteric excipient coatings such as those of the polymethacrylate-type (11-16). This prevents absorption. In the intestine it was now shown in a study with ulcerative colitis patients that supplementation of PC from the luminal side by application of this delayed release lecithin preparation compensates the intrinsic lack of mucus PC (11). Thus, it improves in ulcerative colitis the clinical activity, leads to remission, spares cortisone in steroid-refractory cases and maintains remission (11-16). It may also be of benefit to patients with other inflammatory bowel diseases because it is assumed that the affected patients show a secondary lack of PC in mucus (17,18). Another reason for intestinal susceptibility towards bacterial invasion could be the overgrowth of ectophospholipase containing microbiota thinning the mucus PC content (10,18). Luminally provided PC could satisfy their demand for PC more easily instead of attacking the mucus to release there the less available mucin-2 bound PC, which leads to its depletion in mucus.
The addition of retarding substances such as the methyl acrylate polymer Eudragit S100® causes bloating and renders the formulation as a medication (11-16). For broader availability to the population and reduction of intake quantity, it is intended to use PC without additive as food to maintain colonic health. It is postulated that saturated PC supplementation may indeed be suitable for such an approach. The reason is that due to its physicochemical characteristics it is less accessible to pancreatic phospholipases. It is assumed to have a comparable kinetic profile as an Eudragit S100-covered delayed release formulation of PC.
The maintenance of the intestinal mucus barrier by addition of saturated lecithin to the mucus could be of benefit for patients with ulcerative colitis with intrinsic low PC content in intestinal mucus (2,8). In the present study we evaluated the accessibility of saturated (hydrogenated) PC to pancreatic phospholipases. In case it is less hydrolyzed compared to unsaturated PC, it is less available to the system (low bioavailability) and, thus, remains in intestinal lumen for incorporation in colonic mucus. The clinical efficacy of saturated PC was tested in patients with active ulcerative colitis. Hydrogenated PC mainly consisting of C16:0 and C18:0 PC species was obtained from Lipoid (Ludwigshafen, Germany), a phospholipid producing company. They provide it for cosmetic articles. In USA, it is also used as food additive, e.g., in chocolates, chewing gum, or cake overglaze. The composition of this hydrogenated lecithin preparation is shown in Table 1.
Table 1
| Parameter** | Content | Remarks |
|---|---|---|
| PC (hydrated) | 72% | Measured by HPLC |
| Identity (DC) | Proven | Specific compound detection |
| Lyso-PC (hydrated) | 2–5% | Measured by HPLC |
| Triglyceride | 0.2–3.0% | Determination of substance class |
| Free fatty acids | <0.05–0.5% | Determination of substance class |
| Phosphorus | 3.4–3.7% | Total measurement of phosphorus |
| Iodine count | 1–3 | Titration |
| Water | 0.6–2.0% | Titrimetric determination |
| Heavy metals | – | ICP-MS/ICP-OES |
| Ethanol | <0.1–0.5% | Total measurement of ethanol |
| TAMC | <10–100 cfu/g | Microbial enumeration test |
| TYMC | <10–100 cfu/g | Microbial enumeration test |
| E. coli | Absent | Microbial enumeration test for specified bacteria |
| Salmonella ssp. | Absent | Microbial enumeration test for specific bacteria |
*, hydrogenated phospholipids were prepared from non-genetically modified soybean; **, respective lecithin preparation was a beige powder. PC, phosphatidylcholine; HPLC, high performance liquid chromatography; ICP-MS, inductively coupled plasma mass spectrometry; ICP-OES, inductively coupled plasma optical emission spectrometry; TAMC, total aerobic microbial count; cfu, colony forming units; TYMC, total combined yeast/molds counts.
Methods
Phospholipase activity measurement
Two mg/mL of various PC species [dimyristyl-PC, dioleyl-PC, dipalmitoyl-PC, distearyl-PC (all from Avanti Polar Lipids, Alabaster, USA)] and a mixture of 16:0 and 18:0 PC (Lipoid P 75-3, Lipoid GmbH, Ludwigshafen, Germany) prepared in 10 mM Hepes, 150 mM NaCl, 2 mM CaCl2 (pH 8.0) were exposed to phospholipase A2 (PLA2) (Sigma, Munich, Germany).
The reaction was started by addition of 20 µL PLA2 (1 mg/mL, 600 U/mg) in a total volume of 8 mL. Generated H+ was recorded by a pH-meter.
Prospective clinical observation trial
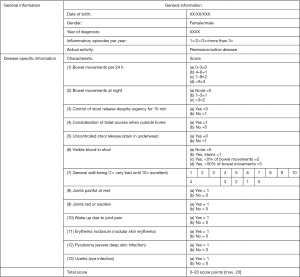
Twenty-four patients with active ulcerative colitis under standard therapy were treated as individual healing attempt orally with 2.8 g hydrogenated lecithin with 70% enriched PC (corresponding to 2.0 g PC) (19). This lecithin Lipoid P 75-3 was obtained from Lipoid, Ludwigshafen, Germany. The patients were evaluated by a questionnaire before and after treatment course (Figure 1).
The saturated PC was orally applied and swallowed with plenty of water on an empty stomach to reduce secretion of pancreatic juice and, thus, prevent degradation by pancreatic phospholipases, a prerequisite for its absorption. As a fluid which rapidly passes the stomach, the release of pancreatic phospholipases by hormonal signaling—as it is known for solid food—is suppressed (20). Thus, the PC is meant to pass in large quantities as intact, non-hydrolyzed form the upper intestine without significant absorption. From the luminal side it may enter then the large intestinal mucus to replenish empty PC binding sites on mucin-2 and, thus, can establish hydrophobicity. This proposed mechanism is experimentally not easily provable, but it is empirically deducted from physiology and earlier studies with intestinally provided PC (7,11).
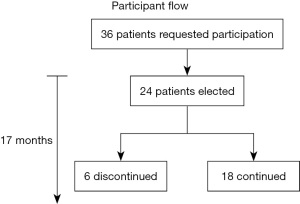
The outcome was evaluated by a questionnaire before and after the treatment course (Figure 1). It focused on inflammatory activity, including bowel movements (24 h and at night), blood in stool, incontinence, achievement of remission, quantification of disease activity improvement (score entry/end) and relief of suffering (score entry/end). Voluntary continuation of lecithin intake was another criterion of efficacy. The duration of therapy was at least 1 month and lasted as long as the patients took the hydrogenated lecithin, which is listed in Table 2. Although patients continued intake, cut-off date was the time when this manuscript was prepared. Inclusion criteria were active ulcerative colitis, willingness to participate and comprehension of the concept of the study. Exclusion criteria were non-active disease and no ulcerative colitis. The participant flow is illustrated in Figure 2. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The trial was performed as individual healing attempt. The protocol was provided on January 24th 2020 to the ethical committee of the Landesärztekammer Baden-Württemberg Stuttgart, Germany. It was declared as a freedom to operate treatment course with the obligation to receive informed consent of the patients after thorough information about background, intention, and risk of the trial. The outcome of the trial was requested to be published.
Table 2
| Characteristics of patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender (F/M) | M | M | F | M | M | M | M | M | M | M | F | M | M | F | M | F | M | F | F | M | M | F | M | M |
| Age (years) | 27 | 39 | 30 | 35 | 35 | 13 | 17 | 32 | 36 | 32 | 20 | 28 | 16 | 45 | 46 | 49 | 33 | 27 | 58 | 22 | 7 | 24 | 42 | 24 |
| Duration ulcerative colitis (years) | 12 | 27 | 12 | 19 | 3 | 1 | 1 | 2 | 11 | 1 | 2 | 1 | 8 | 35 | 32 | 5 | 17 | 9 | 12 | 3 | 2 | 5 | 12 | 6 |
| Extension ulcerative colitis* | p | s | s | l | s | p | p | p | p | p | s | p | p | p | p | s | l | s | p | pr | l | p | l | l |
| Course** | r | r | r | r | r | r | r | r | r | r | r | r | r | r | c | r | r | r | c | r | r | r | r | r |
| Medical history*** | M, S | M, S | M, S, I, B | M, S, I | M, S | M, S, N | M, S, N | S, B, I | M, S, I, B | M, S, I | S, M | M, S, B | M, S | M, S, B, N | N | N | M, S, N | M, S, B, I | M, S, B | S, N | M, N | M, S, B | M, S, N | M, S, B |
| Steroid dependency (Y/N) | Y | N | N | N | N | N | N | N | N | Y | N | N | N | N | N | N | N | N | Y | N | N | N | N | N |
| At entry | ||||||||||||||||||||||||
| Inflammatory activity | ||||||||||||||||||||||||
| Bowel movements (24 h) | 8 | 2 | 14 | 1 | 5 | 2 | 3 | 3 | 3 | 5 | 3 | 4 | 2 | 6 | 9 | 3 | 8 | 10 | 20 | 4 | 2 | 20 | 6 | 4 |
| Bowel movements (night) | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 3 | 0 | 3 | 4 | 3 | 0 | 0 | 3 | 0 | 2 |
| Blood in stool (Y/N) | Y | Y | Y | Y | Y | Y | Y | N | Y | N | Y | Y | N | Y | Y | Y | N | Y | Y | Y | N | Y | N | Y |
| Incontinence (Y/N) | Y | N | Y | N | Y | N | N | N | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | Y | N |
| Under lecithin | ||||||||||||||||||||||||
| Duration of therapy (months) | 17 | 9 | 1 | 7 | 12 | 5 | 14 | 9 | 10 | 14 | 7 | 2 | 8 | 2 | 1 | 1 | 1 | 8 | 4 | 2 | 1 | 8 | 8 | 5 |
| Inflammatory activity | ||||||||||||||||||||||||
| Bowel movements (24 h) | 2 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 3 | 9 | 3 | 4 | 4 | 3 | 3 | 1 | 1 | 1 | 1 |
| Bowel movements (night) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blood in stool (Y/N) | N | N | N | N | N | N | N | N | N | N | Y | N | N | Y | N | Y | N | N | N | N | N | N | N | N |
| Incontinence (Y/N) | N | N | N | N | N | N | N | N | N | N | N | N | N | Y | Y | Y | N | N | N | N | N | N | N | N |
| Remission (Y/N) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | N | N | N | Y | Y | Y | Y | Y | Y |
| Disease activity (score entry/end) | 12/0 | 3/0 | 11/1 | 5/0 | 7/0 | 4/0 | 5/0 | 5/0 | 3/0 | 7/0 | 8/2 | 6/0 | 3/0 | 6/0 | 10/8 | 9/6 | 9/2 | 10/1 | 17/2 | 4/0 | 4/0 | 12/2 | 4/0 | 8/2 |
| Suffering (score entry/end) | 3/0 | 0/0 | 2/0 | 1/0 | 0/0 | 0/0 | 3/0 | 3/0 | 0/0 | 3/0 | 4/1 | 0/0 | 0/0 | 0/0 | 2/2 | 1/1 | 4/1 | 1/0 | 4/2 | 2/0 | 2/0 | 4/2 | 0/0 | 2/0 |
| Continuation of therapy with lecithin (Y/N) | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | N | N | N | Y | Y | Y | N | Y | Y | Y |
| Additional therapy*** | M | M | N | B, I | M, B | N | B | M | M, B, N | N | N | M, S | M, B | N | M, B, N | B | N | B |
*, pr: proctitis, s: sigmoiditis, l: left sided colitis, p: pancolitis; **, c: chronic active disease, r: recurrent disease; *** M: mesalazine, S: steroids; B: biologicals, N: natural medicines and probiotics; I: immunosuppressive drugs.
Statistics
Quantitative results of PLA2 activity were analyzed using the Student’s t-test for comparison between the tested PC species. The t-test was also applied for the paired groups of ulcerative colitis patients before and after PC administration. Two-sided P values were reported and an effect was considered significant at a P value ≤0.05.
Results
First the lack of bioavailability of saturated PC for systemic absorption was tested. It focuses on the accessibility to pancreatic phospholipases. This was proven in the phospholipase activity assay with different PC species. In this analysis the generation of H+ (drop of pH) was recorded based on the reaction shown in Figure 3.
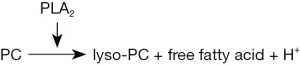
The initial hydrolysis rate of the mixture of 16:0 and 18:0 PC, as it is provided in the applied hydrogenated lecithin preparation corresponded to a pH-drop over 1 min of 0.07±0.02, which was in the range of dipalmitic-PC (0.07±0.02/min) and distearyl-PC (0.04±0.01/min).
The reaction was slow in comparison to dimyristyl-PC with 0.15±0.03/min and dioleyl-PC with 0.1±0.02/min (P values for both <0.05). Thus, saturated long fatty acid containing PCs with at least 15 carbon atoms are less efficiently cleaved by PLA2 compared with PCs with unsaturated fatty acids and PCs with saturated fatty acids with less than 15 carbon atoms.
It is summarized that saturated PC, when provided alone and on an empty stomach as described in “Methods”, prohibits pancreatic enzyme release. Due to its physicochemical properties (melting point, solubility, intermolecular structure), it is less accessible to pancreatic phospholipases and therefore hardly absorbable (21). Thus it could be targeted to colonic mucus. There it is able to fill up empty PC binding sites on mucin-2.
The prospective evaluation of patients participating in an individual treatment attempt demonstrates clinical efficacy of saturated PC
Demographic data
Over a period of 17 months, 24 patients were included in individual treatment attempts with saturated lecithin. Of these 7 were female and 17 were male. The age varied between 7 and 49 years (Table 2).
All of the included patients experienced weight loss before entry. Their disease activity spanned from mild to moderate to severe symptoms. They all had established therapies which did not cure their complaints and, thus, they were therapy refractory cases. Most of them had recurrent disease with an active disease period at the time of trial. Two were chronic active. Extension varied from proctitis to pancolitis with predominance of the last. All of the included patients had to have at least one follow-up examination after 1 month. A continuation of therapy was offered to patients who improved under lecithin and wanted to continue the trial. The application of lecithin was considered as an additive to the conventional therapy. Patients were recommended to maintain their present therapy. Most of them were in the past history on various standard therapies, including mesalazine, steroids, immunosuppressive drugs, biologicals and natural medicines, including probiotics. After informing the patients about the trial procedure and receiving informed consent, the treatment was started with 2×1.4 g saturated lecithin, corresponding to 2 g of PC daily on an empty stomach with at least drinking of 500 mL of still water.
Outcome
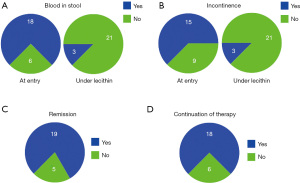
Of the 24 patients treated, all tolerated the intake of hydrogenated lecithin very well without any single reported adverse event. Under PC the total activity score and all subscores dropped significantly (P≤0.05), indicating improvement of disease (Table 2, Figure 4).
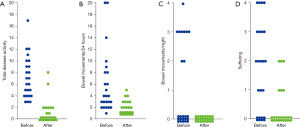
It was the reduction of stool frequency, the disappearance of blood from stool and incontinence, which indicated the beneficial effect of lecithin (Figure 5). In all cases there was an increase in stool consistency noted. Initially elevated Calprotectin levels, which were only available for a few patients dropped to normal values below 46 mg/kg. Three patients who reported joint pain (and swelling), were all improved. Most impressive was the fact that 19 of the patients experienced remission when lecithin was added to the therapy. It represents the switch to a quiescent phase of the recurrent course. The simple fact that most of the patients demanded to be continued on lecithin underlines the efficacy and acceptance of this therapy which was registered by reduction of the suffering score. To be on the safe side, most of the patients maintained a conventional therapy. The five patients who preferred a medication with lecithin alone did this—after respective information—on their own responsibility, but were offered immediate help in case of deterioration.
Discussion
From the observation it is concluded that hydrogenated lecithin is a well-tolerated nutritional adjunctive therapy, which is helpful in suppression of inflammation, as disappearance of blood in stool is the best indicator for mucosal repair. This is supported by the reduction of bowel movement frequency, disappearance of nocturnal stool release, increase of stool consistency, and prevention of incontinence.
Most importantly the wellbeing of the patients was significantly improved. Indeed the overwhelming majority of patients came into remission. New inflammatory episodes were induced by stress situations, e.g., examinations, gastrointestinal infections, or exposure to non-steroidal anti-inflammatory drugs (NSAIDs). However, these intermittently occurring episodes were mild with increased bowel frequency only. No recurrence of active disease over prolonged periods was observed. An indication of the efficacy of the therapy was the adherence of the patients to the intake of saturated PC. Only in 6 of the 24 patients the therapy was interrupted. Reason for discontinuation was lack the of the expected improvement or simply the inconvenience to visit the study center in Baden-Baden due to long distance traveling and/or the corona pandemic. The adherence to therapy was predominant and it was due to the experienced beneficial effects of the therapy and the lack of adverse events. However, the design of the study did not follow the scientific requirements for evaluation of clinical efficacy. This individual healing attempt in 24 patients is rather an observation with bias on patients’ and doctors’ side. Therefore, the results have to be interpreted with caution. Moreover it is an add-on treatment option. The basic therapy was in all cases recommended to be maintained. On later occasions when remission was achieved, the conventional therapies were discussed to be reduced in dosage in agreement with the treating gastroenterologist. Thus, saturated lecithin serves as nutrient for the mucus to maintain a healthy gut. It is not meant to replace conventional therapy.
Anticipated intake of hydrogenated (saturated) lecithin
PC-containing lecithin dietary supplements are already available on the market with an average recommended dose of 3×500 mg. The total dietary intake of PC should be 4.156 g daily, which is based on the required choline supplementation to the organism supposed by the Deutsches Grünes Kreutz e.V., Marburg, Germany (22). Conventional lecithin preparations contain maximally an estimated 5% content of saturated PC. The target of the orally provided saturated PC is the colonic mucus. Earlier dose finding studies with intestinal release lecithin showed clinical improvement at doses between 1–4 g PC daily (13). In adults a dose of 2 g PC daily is effective and safe in regard to provision of a sufficient amount, even under condition of an accelerated passage (11-15). The recommended doses depend on the length of the colon which is age dependent. According to KidsHealth (23), the length of the colon increases from a mean of 52 cm in children aged <2 years to 73 cm at 4–6 years and 95 cm at 9–11 years. Later-on and in adults, the colon is 100–120 cm in length.
Accordingly, from age 9 onwards the full 2 g PC dose daily is recommended. Below the age of 4 years 1 g and between 4–9 years 1.5 g daily is recommended. A maximal dose of 4 g may still be harmless, because luminally provided PC targets the colonic mucus as “extracorporal” compartment and the non-used PC is excreted in the stool (250–300 g stool release per day). The daily dosage can be split in two applications per day, e.g., 2-time 1 g PC. The same dosing regimens apply to females and males.
It is proposed for saturated PC that it has similar biological characteristics of delayed availability to the colon as the previously used encapsulated PC. Due to the presence of saturated fatty acid side chains it can more densely be packed onto the mucin than unsaturated PC and, thus, may provide even more protection. Due to the lack of unsaturated fatty acids it is stable for at least 18 months at room temperature and can therefore be stored outside the refrigerator.
Estimate of exposure to undesirable substances
Lecithin is a commercially available food ingredient/additive since many decades. It originates from acetone extracts of PC-rich food sources with only little hydrophobic peptide and protein contamination. This is of advantage, because allergic reactions are rather unlikely, but may not be excluded. In these cases other sources of lecithin should be considered. Beside the PC, which is important for its beneficial effect in the colon, there are other plant or egg lipophilic residues present in the different lecithin preparations. This co-isolated material has never been reported to be harmful. Saturated PC, prepared by hydrogenation of 70% PC-containing lecithin, is considered safe and used in the United States of America as additive in food and canned food as stabilizer (24). The 30% residues in the here explored 70% PC containing lecithin preparation represent a minor part of the product.
Precautions and restrictions of use
Although there are no hints in human experience described in literature which document adverse events, there are two effects, which have to be considered. Luminally provided PC can be broken down by bacteria to trimethylamine (TMA). This is absorbed and in the liver converted to trimethylamine-N-oxide (TMAO) which is considered an arteriosclerotic/cardiovascular risk factor (25). However, this is still under debate because the bacterial density in small intestine is low in comparison to the colon. Moreover, the passage from duodenum to terminal ileum is rapid, mostly less than 2 h. Thus, only minute amounts of TMA should be produced and absorbed. The amount of TMA generated in colon is significantly higher, but absorption from there is considered marginal (26). Moreover patients with ulcerative colitis are mostly young and arteriosclerosis is in the majority not an issue. Furthermore, other risk factors are more important such as smoking, hypertension or hypercholesterinemia.
The second potential risk is the generation of nitrosamines and the presence of chemical contaminants or toxic minerals accumulating during the production process.
Use of saturated PC for other indications in humans and its prove of non-toxicity
The proposed orally applied saturated PC as nutrient for colonic mucus is comparable to commercially available natural (Alveofact/Survanta/Curosurf) and artificial (Exosurf) surfactant preparations, which contain within the saturated phospholipid fraction 70% dipalmitoyl-PC in addition to 1% low molecular hydrophobic proteins or apoproteins, respectively (27,28). The recommended maximal dose is 400 mg/kg (27,28).
These preparations are provided as intratracheal suspensions and are as such not absorbable to the circulation. Only when the integrity of the alveolar lining is disrupted surfactant may distribute to the systemic circulation (28). Larger studies, however, are not available. They are provided to newborns with respiratory distress syndrome.
For non-absorbable, colonic release lecithin preparations coated with polymethacrylate data on adverse events are now available from more than 500 patients in clinical trials (11-15,29). No toxicology, no adverse events and no side effects extending those observed in the placebo group were detected. The saturated PC was tested within individual healing attempts in 24 patients who did not report any toxicology reaction or adverse events.
Due to its negligible absorption, saturated PC is considered a non-toxic nutrient only for colonic mucus. Excretion of lung and intestinal PC follows the route of mucus to be expectorated with sputum or to be released with the stool, respectively.
Conclusions
Hydrogenated Lecithin is an option for supportive therapy of ulcerative colitis as it functions as protecting additive to colonic mucus. It may also to be helpful in Crohn’s disease due to feeding the ectophospholipase of microbiota, thus, preventing their consumption of protective mucus PC. The option of randomized controlled trials with hydrogenated lecithin is desirable. At least in this trial no adverse events were recorded. This makes it suitable for application as food additive or functional food on top of conventional therapies. The perspective is to provide it as an adjunct for establishment of colonic health.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Medical Journal for the series “Liver Diseases: Symptoms, Causes and Novel Therapies”. The article has undergone external peer review.
Data Sharing Statement: Available at https://amj.amegroups.com/article/view/10.21037/amj-21-25/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://amj.amegroups.com/article/view/10.21037/amj-21-25/coif). The series “Liver Diseases: Symptoms, Causes and Novel Therapies” was commissioned by the editorial office without any funding or sponsorship. WS and RW served as unpaid Guest Editors of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The trial was performed as individual healing attempt. The protocol was provided on January 24th 2020 to the ethical committee of the Landesärztekammer Baden-Württemberg Stuttgart, Germany. It was declared as a freedom to operate treatment course with the obligation to receive informed consent of the patients after thorough information about background, intention, and risk of the trial. The outcome of the trial was requested to be published.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol 1995;57:565-83. [Crossref] [PubMed]
- Braun A, Treede I, Gotthardt D, et al. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis: a clue to pathogenesis. Inflamm Bowel Dis 2009;15:1705-20. [Crossref] [PubMed]
- Agassandian M, Mallampalli RK. Surfactant phospholipid metabolism. Biochim Biophys Acta 2013;1831:612-25. [Crossref] [PubMed]
- Amadei F, Fröhlich B, Stremmel W, et al. Nonclassical Interactions of Phosphatidylcholine with Mucin Protect Intestinal Surfaces: A Microinterferometry Study. Langmuir 2018;34:14046-57. [Crossref] [PubMed]
- Ehehalt R, Braun A, Karner M, et al. Phosphatidylcholine as a constituent in the colonic mucosal barrier--physiological and clinical relevance. Biochim Biophys Acta 2010;1801:983-93. [Crossref] [PubMed]
- Stremmel W, Staffer S, Gan-Schreier H, et al. Phosphatidylcholine passes through lateral tight junctions for paracellular transport to the apical side of the polarized intestinal tumor cell-line CaCo2. Biochim Biophys Acta 2016;1861:1161-9. [Crossref] [PubMed]
- Stremmel W, Staffer S, Schneider MJ, et al. Genetic Mouse Models with Intestinal-Specific Tight Junction Deletion Resemble an Ulcerative Colitis Phenotype. J Crohns Colitis 2017;11:1247-57. [Crossref] [PubMed]
- Ehehalt R, Wagenblast J, Erben G, et al. Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients. A quantitative approach by nanoElectrospray-tandem mass spectrometry. Scand J Gastroenterol 2004;39:737-42. [Crossref] [PubMed]
- Braun A, Schönfeld U, Welsch T, et al. Reduced hydrophobicity of the colonic mucosal surface in ulcerative colitis as a hint at a physicochemical barrier defect. Int J Colorectal Dis 2011;26:989-98. [Crossref] [PubMed]
- Stremmel W, Staffer S, Stuhrmann N, et al. Phospholipase A2 of Microbiota as Pathogenetic Determinant to Induce Inflammatory States in Ulcerative Colitis: Therapeutic Implications of Phospholipase A2 Inhibitors. Inflamm Intest Dis 2018;2:180-7. [Crossref] [PubMed]
- Stremmel W, Merle U, Zahn A, et al. Retarded release phosphatidylcholine benefits patients with chronic active ulcerative colitis. Gut 2005;54:966-71. [Crossref] [PubMed]
- Stremmel W, Ehehalt R, Autschbach F, et al. Phosphatidylcholine for steroid-refractory chronic ulcerative colitis: a randomized trial. Ann Intern Med 2007;147:603-10. [Crossref] [PubMed]
- Stremmel W, Braun A, Hanemann A, et al. Delayed release phosphatidylcholine in chronic-active ulcerative colitis: a randomized, double-blinded, dose finding study. J Clin Gastroenterol 2010;44:e101-7. [Crossref] [PubMed]
- Karner M, Kocjan A, Stein J, et al. First multicenter study of modified release phosphatidylcholine "LT-02" in ulcerative colitis: a randomized, placebo-controlled trial in mesalazine-refractory courses. Am J Gastroenterol 2014;109:1041-51. [Crossref] [PubMed]
- Stremmel W, Hanemann A, Ehehalt R, et al. Phosphatidylcholine (lecithin) and the mucus layer: Evidence of therapeutic efficacy in ulcerative colitis? Dig Dis 2010;28:490-6. [Crossref] [PubMed]
- Stremmel W, Vural H, Evliyaoglu O, et al. Delayed-Release Phosphatidylcholine Is Effective for Treatment of Ulcerative Colitis: A Meta-Analysis. Dig Dis 2021;39:508-15. [Crossref] [PubMed]
- Stremmel W, Ehehalt R, Staffer S, et al. Mucosal protection by phosphatidylcholine. Dig Dis 2012;30:85-91. [Crossref] [PubMed]
- Stremmel W, Lukasova M, Weiskirchen R. The neglected biliary mucus and its phosphatidylcholine content: a putative player in pathogenesis of primary cholangitis-a narrative review article. Ann Transl Med 2021;9:738. [Crossref] [PubMed]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER). Ulcerative colitis: Clinical trial endpoints, Guidance for industry. 2016. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/ulcerative-colitis-clinical-trial-endpoints-guidance-industry (last assessed August 21, 2021).
- Stremmel W. Absorption of fat and fat-solulbe vitamins. In: Caspary WF. editor. Structure and function of the small intestine. Amsterdam: Excerpta Medica, 1987:175-85.
- Small DM. Phase equilibria and structure of dry and hydrated egg lecithin. J Lipid Res 1967;8:551-7. [Crossref] [PubMed]
- Deutsches Grünes Kreuz e.V. Informationsportal für Gesundheit. Available online: https://dgk.de/e-shop.html (last assessed August 21, 2021).
- Nemours KidsHealth. Available online: https://kidshealth.org/ (last assessed August 21, 2021).
- Curosurf. Swissmedic-genehmigte Fachinformation, Chiesa SA, Villard-sur-Glane-Swiss. Available online: https://www.compendium.ch/product/57279-curosurf-install-susp-120-mg-1-5ml/mpro#MPro7000:%202015 (last assessed August 21, 2021).
- Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575-84. [Crossref] [PubMed]
- Stremmel W, Schmidt KV, Schuhmann V, et al. Blood Trimethylamine-N-Oxide Originates from Microbiota Mediated Breakdown of Phosphatidylcholine and Absorption from Small Intestine. PLoS One 2017;12:e0170742. [Crossref] [PubMed]
- Physicians desk reference. Exosurf Neonatal for intratrachael suspension. Available online: https://physicians.aneas.net/medical-productX.asp?i=2093 (last assessed August 21, 2021).
- U.S. Food & Drug Administration. Agency Response Letter GRAS Notice No. GRN000534. Available online: https://www.fda.gov/food/gras-notice-inventory/agency-response-letter-gras-notice-no-grn-000662 (last assessed August 21, 2021).
- Dignass A, Schnabel R, Romatowski J, et al. Efficacy and safety of a novel high-dose mesalazine tablet in mild to moderate active ulcerative colitis: a double-blind, multicentre, randomised trial. United European Gastroenterol J 2018;6:138-47. [Crossref] [PubMed]
Cite this article as: Stremmel W, Fricker G, Weiskirchen R. Saturated phosphatidylcholine as dietary additive for colonic mucus: an open label prospective clinical observation trial. AME Med J 2022;7:10.

